Introduction
Ovarian cancer is the seventh most common cancer,
and it is the most common cause of mortality from gynecological
cancers worldwide, with 238,619 incident cases in 2012 according to
Globocan (http://globocan.iarc.fr/Default.aspx) (1). In developing countries, it is ranked
the second most common gynecological cancer, and constitutes the
fourth most common of all cancers in women, with 17,755 incident
cases in 2012. Essentially, the highest incidence rates of ovarian
cancer are found in the developed countries. Northern Europe has
the highest incidence rate (13.3 per 100,000 person-years),
followed by Western Europe (11.3 per 100,000 person-years) and
Northern America (10.7 per 100,000 person-years), whereas North
Africa has the lowest incidence rate (2.6 per 100,000 person-years)
(2).
The incidence rate of ovarian cancer in the entire
Sudan has yet to be identified; however, in a hospital-based data
set from the National Cancer Institute, Gezira University, Central
Sudan and Radiation Isotopes Center in Khartoum, collected between
2000 and 2006, ovarian cancer accounted for 6.8% (949) of all
recorded cancers (n=226,652), and it was ranked the sixth most
common cancer for both genders (3).
Additionally, in a more recent data set (2009–2010) from the
National Cancer Registry for Khartoum State alone, ovarian cancer
was the fourth most common cancer in women, with an estimated
incidence rate of 188 per 100,000 population, a gender-specific
rate of 8.0 per 100,000 population, and an age-standardized rate
(ASR) of 7.0 per 100,000 population (4). Furthermore, neither the morality rate
for ovarian cancer nor the survival rate in Sudan has previously
been described due to a lack of the availability of death
certificates, the majority of patients presenting with advanced
stage disease were not thoroughly investigated or treated
symptomatically.
Globally, a lack of reliable screening modalities
has restricted the opportunities for early diagnosis and cancer
detection, leading to a significant proportion of women worldwide
presenting at an advanced stage of the disease. Due to this late
presentation, available treatments are ineffective, and the
majority of patients relapse following treatment-induced regression
(5). Furthermore, there is
substantial geographic variation in the incidence of ovarian cancer
and mortality, with higher incidence observed in developed
countries (9.4 per 100,000 women) compared with women living in the
developing world (5.0 per 100,000 women) (6).
The present study aimed to describe the time trends
of the incidence rate of ovarian cancer in women diagnosed in
Central Sudan between the years 2000 and 2011, as well as to
investigate the age at diagnosis, histological type, stage,
management and survival pattern of women with ovarian cancer
presenting at the National Cancer Institute, Gezira University
(NCI-UG), Sudan.
Materials and methods
The present study reports on patients with ovarian
cancer who received treatment at NCI-UG, Wad Madani, Gezira State,
Sudan, between January 2000 and December 2011. The NCI-UG offers
gynecologic-oncological services to residents of Gezira State, as
well as other neighboring states. All cases were referred to the
NCI-UG, as it the only cancer center in the region to offer cancer
treatment, as mentioned above. The Gezira, or Al Jazirah, State is
located in central Sudan, south of the capital city, Khartoum. The
present study was conducted with the approval of the NCI-UG's and
Purdue University's Institutional Review Boards.
During the study period, Sudan was recognized as the
largest country in the African continent, with a total area of
96,710 square miles, making the country slightly larger than
one-quarter the size of the USA. Sudan is divided into 26 states
and districts, with differing population densities. The states'
Ministries of Health, Armed Forces, Universities, Police and
private sector collectively, in an uncoordinated manner, provide
health services to the people of Sudan. The public sector health
services in Sudan are organized at three levels: Primary, secondary
and tertiary. The states' general hospitals are the referral
centers for the entire state (see the Federal Ministry of Health's
website: http://ghdx.healthdata.org/organizations/federal-ministry-health-sudan).
The Radiation and Isotope Center in Khartoum (RICK) and NCI-UG at
Wad Madani, Gezira State are the only two specialized cancer
centers providing chemotherapy and radiotherapy services for all 26
states. Treatment is offered free for cancer patients. After
exhausting all medical attempts at treatment at the primary and
secondary care facilities, as well as local healers, patients are
referred to RICK or NCI-GU, depending on the proximity to the
patient's residence.
For the present study, data on the age of the
patient at diagnosis, tumor pathology, stage of disease, treatment
given, as well as demographic information (occupation, tribal
affiliation), patient residence (urban/rural) and area of
residence, were retrieved from the NCI-UG Cancer registry. The
NCI-UG Cancer registry was the first national cancer registry to be
established in Sudan (in 2006) with the support of the
International Agency for Research (IARC). It uses the CanReg format
(www.iacr.com.fr/canreg5.htm). Data
are collected actively, as well as passively, and are checked for
accuracy prior to being entered in the computer. Patients with a
diagnosis of ovarian cancer from all gynecological hospitals in
Gezira State and the surrounding states are referred to the NCI-UG.
Usually, the referred patients will have a pathology or cytology
report. In rare cases, for patients who were not fit for surgery or
who had a negative cytology report, a clinical diagnosis was used
[clinical presentation or imaging, in addition to an assessment of
the tumor biomarker, cancer antigen 125 (CA125)].
Tumors were classified according to the tumor-lymph
node-metastasis (TNM) classification, which is based on size of the
primary tumor and presence of metastatic regional lymph nodes
and/or of distant metastases (7).
Few data on grade were available (note that this was not a common
service at these local facilities). Tumors were graded as poorly
differentiated, moderately differentiated or well differentiated.
Staging was based on the primary operative report, and was
performed according to the systems adopted by the International
Federation of Gynecology and Obstetrics (FIGO) (8). Histological types of tumors were coded
according to the International Classification of Diseases for
Oncology (ICD-O) (9). The specific
subtypes of tumors were as follows: Carcinoma, not otherwise
specified (NOS), clear cell, papillary serous cystadenocarcinoma
(PSCAD), moderately differentiated papillary serous adenocarcinoma
(MDPSAD), moderately differentiated mucinous adenocarcinoma
(MDMAD), endometrioid carcinoma (END/ENDOM), sex cord tumors (SCT),
germ cell tumors (GCT) and mucinous cystadenocarcinoma (MCAD).
Patients were treated with surgery, and preoperative
and postoperative chemotherapy. Patients were followed every 2
months during the first year, every 3 months during the second
year, 4 months during the third year, and subsequently 6 months
thereafter. During follow-up, patients were evaluated according to
their history and standard diagnostic investigations, if
required.
Statistical analysis
Descriptive statistics were presented to summarize
the distribution of the demographics of the study sample. Different
incidence rates of ovarian cancer, including the annual incidence
rate, the age-specific rate and the ASR, were derived (10). The direct method was used to compute
ASR using different standard populations, including the 2008 Sudan
population and the 1966 and 2000 World Standard Populations (WSPs).
The Kaplan-Meier method was used to estimate survival functions and
median survival time. Log-rank tests were used to statistically
compare between the survival functions. IBM SPSS Statistics for
Windows, version 20.0 (IBM SPSS, Armonk, NY, USA) was used to
analyze the data. P<0.05 was taken to indicate a statistically
significant value.
Results
Demographic data
During the period between January 2000 and December
2011, 341 ovarian cancer cases were diagnosed at the NCI-UG Center.
Table I shows the distribution of
ovarian cancers based on patient characteristics. The ages of the
women at diagnosis ranged between 9 and 90 years, and the median
age was 50 (standard deviation, ±16 years). Of the 341 women
diagnosed with ovarian cancer, 239/341 (70%) were from the Gezira
State, whereas the remaining 30% were from the surrounding states,
with the majority from Sinnar. Patients with ovarian cancer
belonged to 40 tribal affiliations, with the majority belonging to
Jaaleen (59/341; 17%), Kawahla (38/341; 11%), Rhfaa (32/341; 9%)
and Shaiqiah (22/341; 6%).
 | Table I.Distribution of ovarian cancers based
on patient characteristics. |
Table I.
Distribution of ovarian cancers based
on patient characteristics.
| Variable |
Frequencya
(%) |
|---|
| Age total | 340 (100) |
|
<15 | 3 (1) |
|
15–15 | 16 (5) |
|
25–54 | 166 (49) |
|
55–64 | 75 (22) |
| 65+ | 80 (23) |
| Stage total | 341 (100) |
| 0 | 4 (1.2) |
| I | 57 (1.7) |
| II | 32 (9.4) |
| III | 169 (49.6) |
| IV | 79 (23.2) |
| Age-specific rate
(per 100,000) |
|
|
<15 | 0.42 |
|
15–24 | 4.13 |
|
25–54 | 27.21 |
|
55–64 | 109.34 |
| 65+ | 111.08 |
| Occupation | 340 (100) |
|
Child | 2 (1) |
|
Employee | 19 (5.5) |
| Farmer
and worker | 20 (5.8) |
|
Housewife | 278 (82) |
| Residence | 340 (100) |
|
Rural | 262 (77) |
|
Urban | 78 (23) |
Tumor histopathology
Histological classification of ovarian tumors for
the 341 patients diagnosed with ovarian cancer at the NCI-UG is
shown in Fig. 1. Germ cell tumors
predominantly affected younger women, aged <50 years old (25/30;
83%), and predominantly stage 1 patients (50%).
Tumor stage
According to the TNM staging criteria, the majority
of patients (73%) were diagnosed with late-stage (stage III and IV)
cancer (Table I).
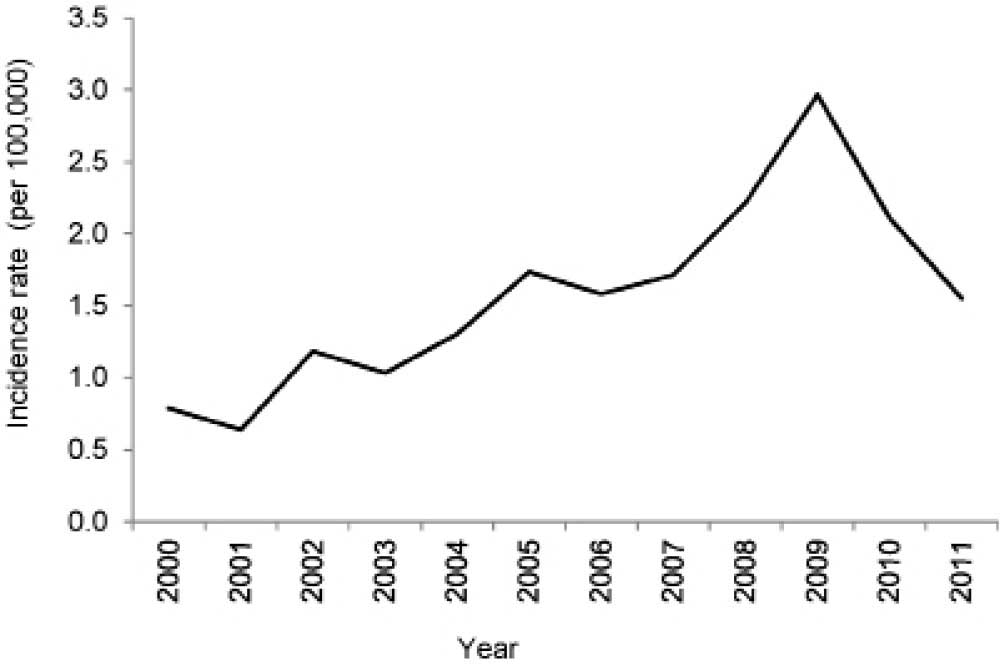
Incidence of ovarian cancer
An increasing trend in the incidence rate of ovarian
cancer was observed between 2000 and 2009, after which time it
started declining in 2010 and 2011 (Fig.
2). The annual incidence rate peaked at 3 per 100,000 women in
2009. The ASRs on a yearly basis are presented in Fig. 3. The cumulative ASRs between the
years 2000 and 2011 were measured as 22, 27 and 30 per 100,000
women using the 2008 Sudan population, the 1966 WSP and the 2000
WSP as the standard population, respectively. Similarly, the ASR
increased between 2000 and 2009, and started to decline in 2010 and
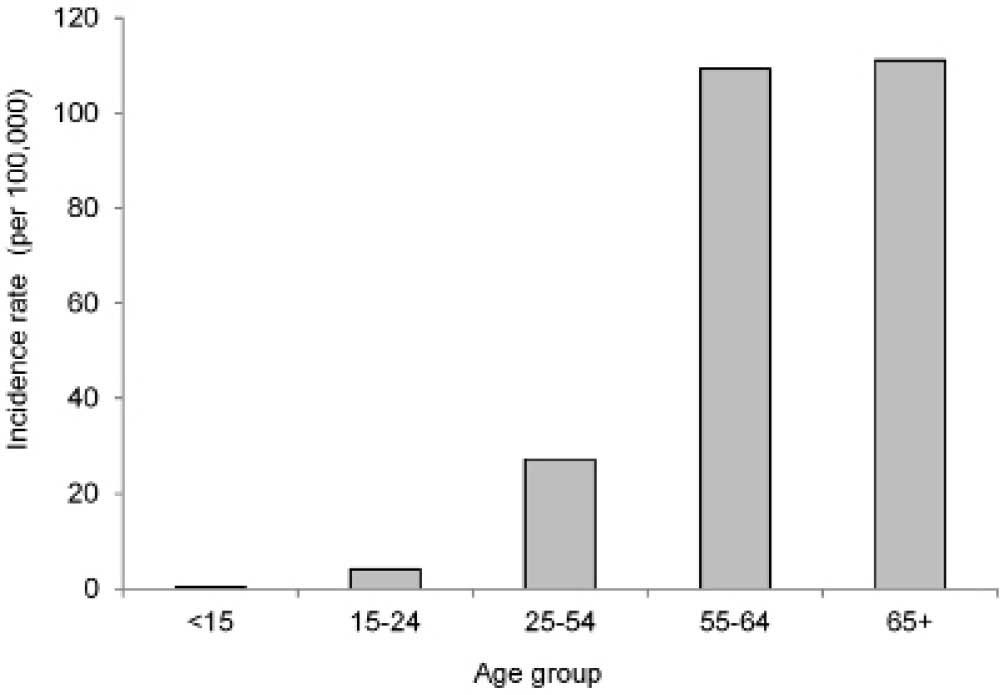
2011 (Fig. 3). The incidence rate of
ovarian cancer increased greatly in women aged 55 years or older
(Fig. 4).
Surgical and chemotherapy
management
Treatment consisted of a primary operation, followed
by chemotherapy and/or radiotherapy. The standard surgical
procedure at the NCI-UG and Wad Medani Obstetrics and Gynecology
Hospital included total abdominal hysterectomy, bilateral
salpingo-ophrectomy and omentectomy, which was performed in 37% of
the cases. Other surgical procedures carried out by gynecologists
outside of Wad Madani included salpingo-ophrectomy, bilateral
salpingo-ophrectomy and partial surgery (performed in 7, 0.6 and
18% of the patients, respectively). Patients who were not fit for
an operation were diagnosed using fine-needle aspiration cytology
(17%) and or tru-cut biopsy (35%) and tumor marker (CA125)
evaluation. Cases in which an operation was not possible, due to
advanced disease, metastatic disease or the patient being unsuited
for an operation, received neoadjuvant chemotherapy prior to a
re-evaluation for complete surgery. Patients who underwent partial
surgery prior to visiting the NCI-UG received either complete
surgery or neoadjuvant chemotherapy first, and subsequently
interval debulking surgery, depending on the response to
chemotherapy and the patient's fitness.
Chemotherapy, used as a neoadjuvant therapy or
post-operatively as an adjuvant therapy, depends on the stage and
grade of the cancer, as per international guidelines (11). All patients (with the exception of
stage I patients) received adjuvant chemotherapy. For stage I
patients, only those with a high-grade tumor and positive cytology
received adjuvant chemotherapy. The chemotherapy administered was
carboplatin-based (carboplatin/cyclophosphamide), and patients who
had a poor response, or who suffered a relapse shortly after the
chemotherapy, received second-line taxol-base chemotherapy
(taxol/carboplatin). However, the taxol supply was not sustainable,
due to financial reasons and also availability, and therefore
Taxol/carboplatin could only be used as a second-line treatment.
Approximately 27% (92/341) of patients with serous
cystadenocarcinoma, or cancer not otherwise specified, received
preoperative chemotherapy and, of those, 48% (44/92) received
postoperative chemotherapy. Approximately 73% (249/341) of the
patients received no preoperative chemotherapy; of those, 10% had
germ cell tumors, 50% had serous cystadenocarcinoma, and 16% had
cancer not otherwise specified. Of those 249 patients, 16% received
no postoperative chemotherapy.
Survival rate of patients with ovarian
cancer
Of the 341 patients diagnosed at the NCI-UG Center
between January 2000 and December 2011, 64/341 (19%) were still
alive, 39% were lost to follow up, and 42% had succumbed to
mortality. The median survival time was 31 months [95% confidence
interval (CI), 19–43]. The 5-year cumulative survival rate of
patients with ovarian cancer in Gezira State was 38% (95% CI,
30–46%). The cumulative survival rate was significantly different
among the disease stages at the time of diagnosis (P<0.001). The
median survival time in the stage III and IV patients was 23 (95%
CI, 18–28) and 15 (95% CI, 3–27) months, respectively (Fig. 5A). Fig.
5B shows survival curves for patients living in urban or rural
areas.
Discussion
The current study has presented data collected from
women with ovarian cancer who visited the NCI-UG in Gezira State
for diagnosis and treatment. Data on 341 patients were analyzed for
the period between January 2000 and December 2011. Within this
group of women, the median age at diagnosis was 50 years. Similarly
young ages for the onset of cancer have been reported for women
from other sub-Saharan African (12–14) and
other (15) developing countries.
However, it is different from that reported for developed
countries, in which the median age at diagnosis is 61 years
(16,17). This may indicate that ovarian cancer
affects young women in Africa. However, this observation of a
younger age at diagnosis in African countries compared with other
developed countries is likely to be due to the difference in
population age distribution between the two: Africa has, by far,
the youngest population of any of the continents (18). Given that age is the single
substantive risk factor for the majority of cancers, including
ovarian cancer, a younger population will have a lower overall
incidence of ovarian cancer and a lower median age of onset, based
simply on the demographics of the population. In the present study,
the age-specific incidence rate, derived from a consideration of
the population size of a particular age group, was determined to be
highest in the group comprising women aged ≥55 years, i.e.
postmenopausal women (Fig. 4), which
is consistent with findings worldwide.
The present study has demonstrated that the majority
of patients with ovarian cancer presented with late stage disease,
i.e. stage III and IV. This late presentation of ovarian cancer is
also observed in other sub-Saharan African countries (12,19).
This finding could be explained by the ‘silent’ nature of the
disease and its non-specific symptoms that hinder early diagnosis,
in addition to a lack of cancer awareness and education, the
influence of local healers and witchcraft, and so forth.
Additionally, the majority of patients live in rural areas, where
transportation and financial affordability may also be influencing
factors. More than 90% of the tumors were epithelial in origin,
with a higher prevalence in older women. Germ cell tumors
constituted 9% of all tumors, and these were mostly predominant in
younger women, i.e. <40 years old (range: 16–39), a similar
presentation to what has been evidenced in the developed countries
(20).
The present study revealed a gradual increase in the
annual incidence rate of ovarian cancer between the years 2000 and
2009, and a subsequent decline in the years 2010 and 2011 in Gezira
State (Fig. 2). The increase
observed during the first nine years in the incidence rate may be
attributed to improvements in diagnostics and treatment services,
due to the establishment of the NCI-UG institution, which is the
first population-based cancer registry in the country in Gezira
State. On the other hand, the decline in incidence rates may be a
result of errors made in the data collection, or it may reflect a
true decrease in the ovarian cancer incident rate. Similar declines
in the incidence rates of ovarian cancer have also been observed in
Northern Europe, USA and China (12–24). An
analysis of the data for 2012–2014 may assist in providing a more
accurate picture regarding the trend in the incidence of ovarian
cancer in Gezira State.
The world population age distribution has changed
considerably, and therefore the World Health Organization (WHO)
introduced, in the year 2000, a new WSP for the calculation of ASRs
for future reports (25) to reflect
more closely the age composition of the world's population, as
projected over the subsequent 25 years. The goal of the new
standard population is to minimize the disproportionate weighting
of events in both the youngest and the oldest population groups
(26). Using this new standard
population, the computed ASRs of a number of cancer types,
including ovarian cancer, are greatly increased (27). However, as the majority of previously
published studies used the 1966 WSP to compute ASRs, they cannot be
compared directly with the ASRs computed using the 2000 WSP.
Therefore, in the present study, both 1966 and 2000 WSPs were used
to calculate the ASR for ovarian cancer. The 2000–2011 cumulative
ASRs using the 2008 Sudan population, 1966 WSP and 2000 WSP as
standard population were determined to be 22, 27 and 30 per 100,000
women, respectively. The annual ASRs, using the 2008 Sudan
population and WSPs 1966 and 2000 as standard populations, were
2.7, 3.3 and 3.7 per 100,000 women, respectively. Based on Globocan
data (1) for 2008 using the 1966
WSP, the reported 3.3 per 100,000 ASR for Gezira State is lower
compared with the ASR of all ovarian cancers reported worldwide
(6.1 per 100,000), and with that of the entire Sudan (6.4), Africa
(4.8), Sub-Saharan Africa (4.6), Eastern Africa (5.5) and Northern
Africa (5.6). The highest ASRs reported for ovarian cancer were
from the USA (8.1), all Europe (9.9) and Australia and New Zealand
(7.6) (27). The increase in ovarian
cancer in the developed world (USA, Europe and Australia and New
Zealand) compared with sub-Saharan Africa may be accounted for by
certain environmental factors and increased cancer awareness,
combined with improved detection strategies.
At NCI-UG and the Wad Medani Obstetrics and
Gynecology Hospital, ovarian cancers are managed by surgical
debulking or cytoreduction of the tumor mass, followed by
combination carboplatin-based chemotherapy, as per international
guidelines. Treatments were administered according to the patients'
age, disease stage, tumor histology and expected pregnancy.
Surgical debulking or cytoreduction for advanced stage disease has
long been shown to increase survival rates. In a meta-analysis of
53 studies of 6,885 patients with stage III and IV disease, it was
reported that every 10% increase in surgical debulking is
associated with a 5.5% increase in the median survival rate
(28). The majority of patients
(45%) in the present study had surgical staging and debulking.
Notably, of those patients, one-third were alive, one third had
succumbed to mortality, and the remaining third were lost to follow
up, preventing the formulation of any conclusions regarding the
survival advantage of surgery in the current study.
Approximately 40% of the patients were lost during
follow up in the present study. Similar percentages of patients
lost to follow up were also observed in Ibadan (29) and Lagos, Nigeria (13). This issue is of concern specifically
in the developing world in determining cancer survival rates, and
has been discussed extensively by Sankaranarayanan et al
(30). In the current study,
Kaplan-Meier curves were used to determine survival rates and
median survival times. Only the time up to the last visit of the
patient was used in the computation of these survival measures. The
loss to follow up may have resulted in a decrease in the
reliability of the estimates, which would have been reflected in
the 95% CI estimates. Without further information on these patients
following their last visit, it would not be possible to accurately
predict any direct bias, if any, in the estimates. However, given
that these patients did not follow through the recommended
treatments, it would be expected that the survival measures
estimated using the available data could have been
overestimated.
In the present study, administration of neoadjuvant
therapy did not alter the overall survival rate of the patients
with ovarian cancer after adjusting for the disease stage at
diagnosis (P=0.734). However, following stratification by disease
stage, administration of adjuvant chemotherapy revealed effects on
prolonging the survival rate in the stage III and IV patients
(P=0.002 and P<0.001, respectively), but did not reveal any
effects in the patients who were diagnosed at an earlier disease
stage. The current study did not identify any additional survival
benefits of neoadjuvant therapy compared with standard treatments
of advanced ovarian cancer, as previously reported by others
(31,32).
A crossover of the survival functions at ~40 months
was observed while comparing between the patients from rural and
urban areas (Fig. 5B). Furthermore,
based on a stratified analysis, no marked difference in the
survival rate was identified between patients from rural (median
survival time, 19 months) and urban (median survival time, 15
months) areas during the 40 months following the diagnosis
(P=0.267). However, after this time point, the patients from urban
areas (median survival time, 121 months) survived longer compared
with the patients from rural areas (median survival time, 96
months), although the difference was not significant (P=0.062). The
5-year cumulative survival rate of patients with ovarian cancer in
Gezira State (38%) was an improvement compared with those reported
for different parts of Nigeria (29–33).
However, the 5-year survival rate was lower compared with the
United States, which may be explained by a lack of awareness and
timely access to health care.
In addition, the current study has demonstrated that
the FIGO stage was an independent prognostic factor for invasive
ovarian cancer. The 5-year survival rate was greatly improved for
early stage cancer compared with the advanced disease.
One limitation of the present study was the
possibility of various errors in data collection and reporting,
which may have occurred, particularly with data collected prior to
the establishment of the cancer registry at NCI-UG. Another
possible limitation of this study was that it did not include
patients who may have left NCI-UG and were subsequently treated at
the Khartoum Radiation and Isotope Center.
In Gezira State, the incidence rate for ovarian
cancer exhibited an increasing trend during the period between 2000
and 2009. It is more common in postmenopausal women, which is in
agreement with reports from the developed world (6). Women presented at an advanced stage of
the disease, which resulted in short survival times. To the best of
our knowledge, this is the first study to determine the survival
time for women with ovarian cancer in the entire Sudan, and it
should serve as a basis from which to monitor the effectiveness of
ovarian cancer management in Sudan in the future.
Acknowledgements
The National Cancer Institute, University of Gezira
supported the Study.
References
|
1
|
Ferlay J, Ervik M, Dikshit R, Eser S,
Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: IARC CancerBase No. 11.
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohammed ME, Hassan AM, Abdelhadi HA,
Elsadig MG, Adam DM, Elmamoun K, Hamid R, Elias H, Abdallah M,
Abdelkarim Z, Elwali NE and Mohammed SI: Burden and pattern of
cancer in the Sudan, 2000–2006. Br J Med Med Res:. 4:1231–1243.
2014. View Article : Google Scholar
|
|
4
|
Saeed IE, Weng HY, Mohamed KH and Mohammed
SI: Cancer incidence in Khartoum, Sudan: First results from the
cancer registry, 2009–2010. Cancer Med. 3:1075–1084. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morrow M: American Joint Committee on
Cancer: AJCC Cancer Staging Manual. 6th. Springer; New York, NY:
pp. 157–164. 2002
|
|
8
|
Shepherd JH: Revised FIGO staging for
gynaecological cancer. Br J Obstet Gynaecol. 96:889–892. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fritz AG: International classification of
diseases for oncology: ICD-O. World Health Organization. 2000.
|
|
10
|
Naing NN: Easy way to learn
standardization: direct and indirect methods. Malays J Med Sci.
7:10–5. 2000.PubMed/NCBI
|
|
11
|
Morgan RJJ, Alvarez RD, Armstrong DK, et
al: NCCN clinical practice guidelines in oncology: epithelial
ovarian cancer. J Natl Compr Cancer Netw. 9:82–113. 2011.
|
|
12
|
Vanderpuye V and Yarney J: Ovarian cancer:
An analysis of forty-four patients at the national radiotherapy
centre, Accra-Ghana. West Afr J Med. 26:93–96. 2007.PubMed/NCBI
|
|
13
|
Rabiu KA, Akinola OI, Adewunmi AA, Fabamwo
AO, Adedeji MO and Popoola AO: Delays in presentation and
management of ovarian cancer in Lagos, Nigeria. J Obstet Gynaecol.
33:305–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doh AS and Shasha W: A
clinico-pathological study of ovarian tumors in Yaounde-Cameroun.
West Afr J Med. 13:196–199. 1994.PubMed/NCBI
|
|
15
|
Basu P, De P, Mandal S, Ray K and Biswas
J: Study of ‘patterns of care’ of ovarian cancer patients in a
specialized cancer institute in Kolkata, eastern India. Indian J
Cancer. 46:28–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quirk JT and Natarajan N: Ovarian cancer
incidence in the United States, 1992–1999. Gynecol Oncol.
97:519–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Altena AM, Karim-Kos HE, de Vries E,
Kruitwagen RF, Massuger LF and Kiemeney LA: Trends in therapy and
survival of advanced stage epithelial ovarian cancer patients in
the Netherlands. Gynecol Oncol. 125:649–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harford JB: Breast-cancer early detection
in low-income and middle-income countries: Do what you can versus
one size fits all. Lancet Oncol. 12:306–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gharoro EP and Eirewele O: Cancer of the
ovary at the university of benin teaching hospital: A 10-year
review, 1992–2001. Afr J Med Med Sci. 35:143–147. 2006.PubMed/NCBI
|
|
20
|
Sankaranarayanan R and Ferlay J: Worldwide
burden of gynaecological cancer: The size of the problem. Best
Pract Res Clin Obstet Gynaecol. 20:207–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bray F, Loos AH, Tognazzo S and La Vecchia
C: Ovarian cancer in Europe: Cross-sectional trends in incidence
and mortality in 28 countries, 1953–2000. Int J Cancer.
113:977–990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris CR, Rodriguez AO, Epstein J and
Cress RD: Declining trends of epithelial ovarian cancer in
California. Gynecol Oncol. 108:207–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lowe KA, Chia VM, Taylor A, O'Malley C,
Kelsh M, Mohamed M, Mowat FS and Goff B: An international
assessment of ovarian cancer incidence and mortality. Gynecol
Oncol. 130:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong KH, Mang OW, Au KH and Law SC:
Incidence, mortality, and survival trends of ovarian cancer in Hong
Kong, 1997 to 2006: A population-based study. Hong Kong Med J.
18:466–474. 2012.PubMed/NCBI
|
|
25
|
Ahmad OBB-PC, Lopez AD, Murray CJL, Lozano
R and Inoue M: Age standardization of rates: A new WHO
standardGlobal programme on evidence for health policy discussion
paper Series: no. 31. Geneva: 2000
|
|
26
|
Bray F, Guilloux A, Sankila R and Parkin
DM: Practical implications of imposing a new world standard
population. Cancer Causes Control. 13:175–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bristow RE, Tomacruz RS, Armstrong DK,
Trimble EL and Montz F: Survival effect of maximal cytoreductive
surgery for advanced ovarian carcinoma during the platinum era: A
meta-analysis. J Clin Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adekanbi AA, Olayemi O, Okolo CA, Fawole
AO, Odukogbe AT and Okani CO: Survival of ovarian cancer patients
in Ibadan: Clinical and pathological factors. J Obstet Gynaecol.
34:57–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sankaranarayanan R, Black RJ, Swaminathan
R and Parkin DM: An overview of cancer survival in developing
countries. IARC Sci Publ. 135–157. 1998.PubMed/NCBI
|
|
31
|
Tangjitgamol S, Manusirivithaya S,
Laopaiboon M, Lumbiganon P and Bryant A: Interval debulking surgery
for advanced epithelial ovarian cancer. Cochrane Database Syst Rev:
CD006014. 2016. View Article : Google Scholar
|
|
32
|
Bristow RE, Eisenhauer EL, Santillan A and
Chi DS: Delaying the primary surgical effort for advanced ovarian
cancer: A systematic review of neoadjuvant chemotherapy and
interval cytoreduction. Gynecol Oncol. 104:480–490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iyoke C, Ugwu G, Ezugwu E, Onah N, Ugwu O
and Okafor O: Incidence, pattern and management of ovarian cancer
at a tertiary medical center in Enugu, South East Nigeria. Ann Med
Health Sci Res. 3:417–421. 2013. View Article : Google Scholar : PubMed/NCBI
|