Introduction
Intravenous leiomyomatosis is a rare disease
originating from myometrial veins, characterized by intravascular
nodular masses histologically composed of benign smooth muscle
cells; the masses may extend to a variable distance into the
inferior vena cava, right atrium and even the right ventricle. It
is reported that >50% of the patients have a prior history of
hysterectomy (1). These nodules may
extend into the atrium and/or ventricle and may be accompanied by
clinical symptoms such as breathlessness, pain and congestive
myocardial infarction, and they may be misdiagnosed as cardiac
myxoma. To date, the treatments for intravenous leiomyomatosis
include expectant treatment and surgical resection.
Gonadotropin-releasing hormone analogue (GnRHa) is important in
expectant treatment. However, the outcome of such cases is
generally not satisfactory. Surgical management includes complete
and incomplete resection. We here in present a case of diffuse
uterine leiomyomatosis extending to the right atrium, which was
successfully resected under non-extracorporeal circulation.
Case report
A 39-year-old woman, gravida 2, para 2, presented
with complaints of intermittent abdominal pain over the last 3
years, with palpitations and tightness of the chest over the last 2
months.
In March, 2012, the patient underwent uterine
myomectomy in the Jiangxi Provincial People's Hospital. In January,
2013, the patient was admitted to the Chenzhou City People's
Hospital and underwent panhysterectomy with left adnexectomy.
Detailed information on the abovementioned surgeries were not
available. One year later, the patient presented to the Chenzhou
First People's Hospital due to a recurrent abdominal mass with
abdominal distention, was diagnosed with disseminated intravenous
leiomyomatosis, and three cycles of GnRHa were administered. In
May, 2014, the patient presented to the Peking University Shenzhen
Hospital (Shenzen, China) for treatment, and was prescribed
triptorelin acetate injections (3.75 mg) every 28 days. However,
the patient did not follow the doctor's instructions and the
injections were performed in May, June, August and October. In
December, 2014, the symptoms were aggravated and were accompanied
by edema of the bilateral lower extremities. On general physical
examination, the mass occupied the entire pelvis, resembling a
4-month pregnancy. The patient was again administered
3.75-mgtriptorelin acetate injections in December, 2014 and
January, March and April, 2015. The patient has received a total of
11 cycles of GnRHa to date.
The patient presented to our hospital with a history
of chest tightness and palpitations for 2 months. On physical
examination, a grade III systolic murmur was audible along the left
edge of the sternum. Pelvic examination revealed bilateral pelvic
solid masses sized 10×10 (left side) and 3×4 (right side) cm, hard
on palpation, irregular, immobile and non-tender; the left mass had
reached the left pelvic wall and was fixed to the obturator
foramen. As the patient had first presented to our hospital,
gynecological ultrasonography examination revealed the changes in
the pelvic mass (Table I). Computed
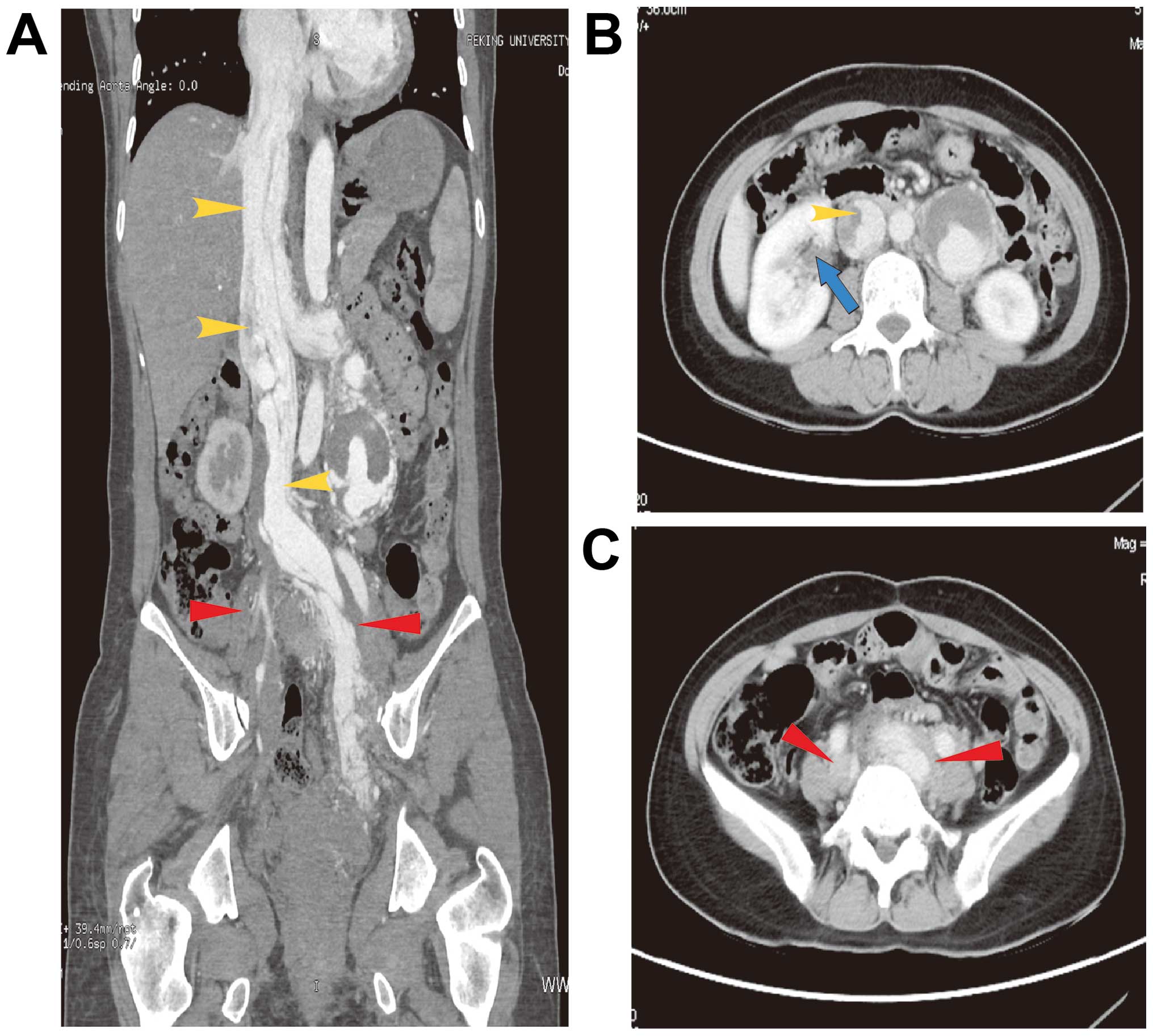
tomography scans revealed pelvic mass extension to the bilateral
internal and common iliac veins, left renal vein and inferior vena
cava, with a small-to-low-density lesion in the liver (Fig. 1). Three-dimensional (3D) cardiac
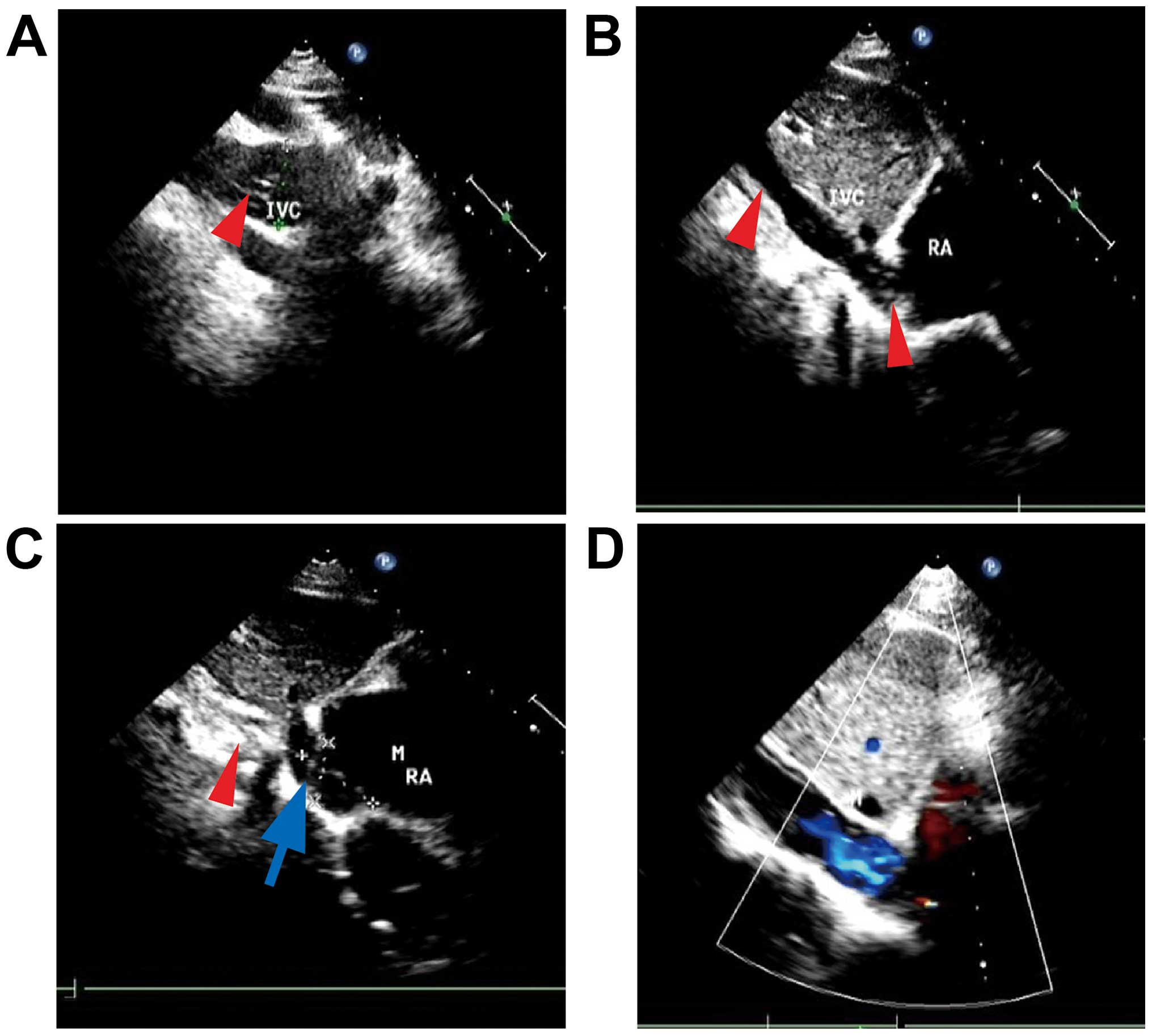
ultrasonography revealed that the wide inner diameter of the
inferior vena cava was 28 mm, with a diffuse mixed echo; a low echo
was mainly visible in the inferior vena cava (unclear boundary,
irregular shape, with strong streak-like echogenicity), partly
extending to the right atrium (28×24 mm), with a small amount of
tricuspid valve regurgitation (Fig.
2A-C).
 | Table I.Results of gynecological
ultrasonography examination of the patient in our hospital. |
Table I.
Results of gynecological
ultrasonography examination of the patient in our hospital.
| Date
(year/month) | Pelvic mass size
(mm) | Mass description |
|---|
| 2014/03 | 72×45×68 | Dispersive
distribution, different sizes |
| 2014/07 | 68×53 | Mixed mass |
| 2014/10 | 81×76×64 | Mixed mass |
| 2014/12 | 120×110 | Multiple hyperechoic
masses, blood flow signals |
| 2015/04 | 104×67×100 | Multiple mass
integration, irregular shape, streak blood flow signals |
Based on the patient's history and the
abovementioned examinations, intravenous leiomyomatosis was
diagnosed. Following a multidisciplinary discussion, the patient
underwent myomectomy (right atrium, inferior vena cava, renal veins
and pelvis), right oophorectomy and pelvic adhesiolysis under
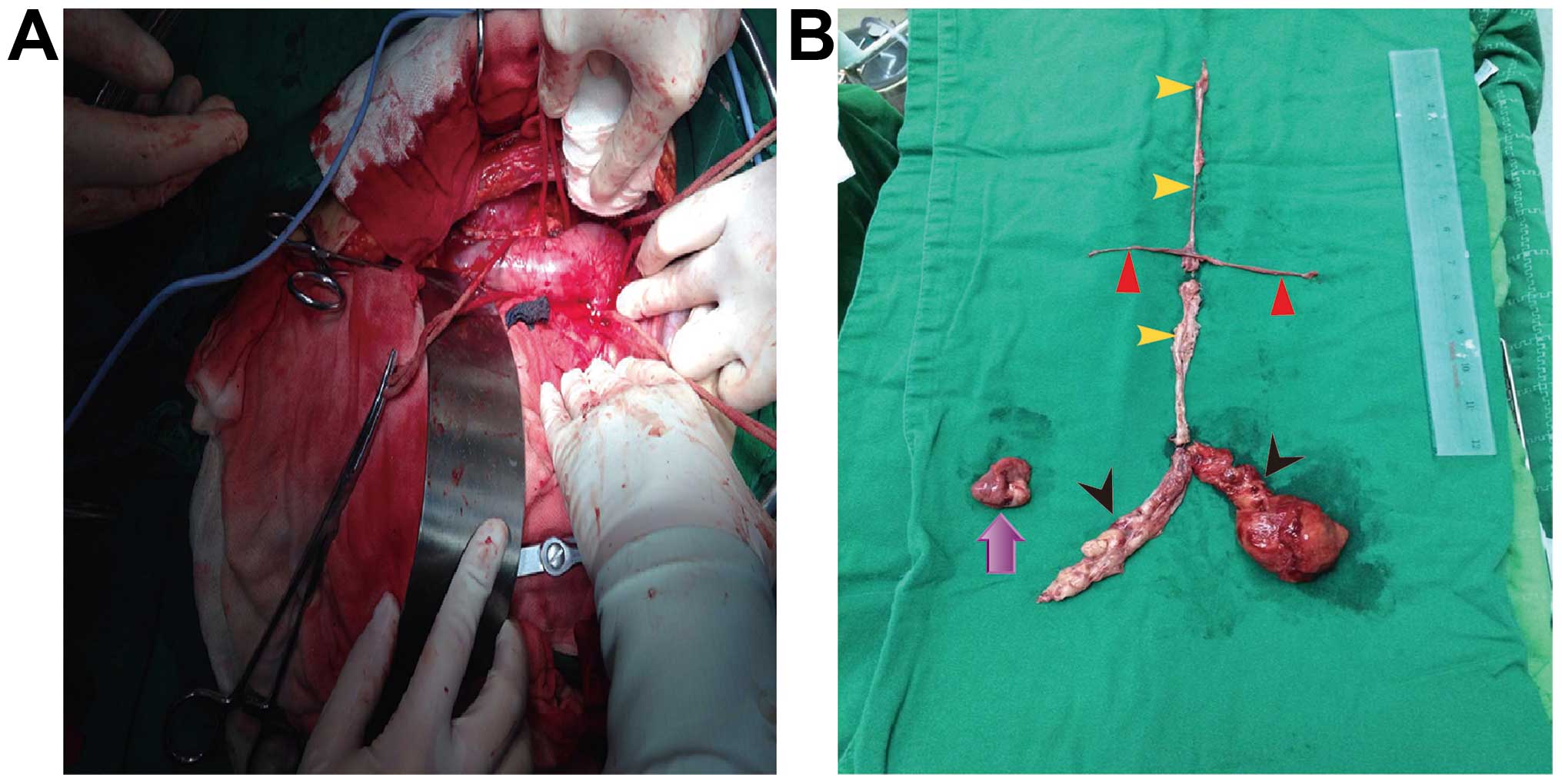
non-extracorporeal circulation. The length of the tumor from the
inferior vena cava to the right atrium was 30 cm; the tumors in the
bilateral renal veins were 6 and 5 cm, respectively; the tumor in
the right common iliac vein was sized 12×4 cm; the tumor in the
left common iliac vein to the left pelvis was 8×7 and 10 cm in
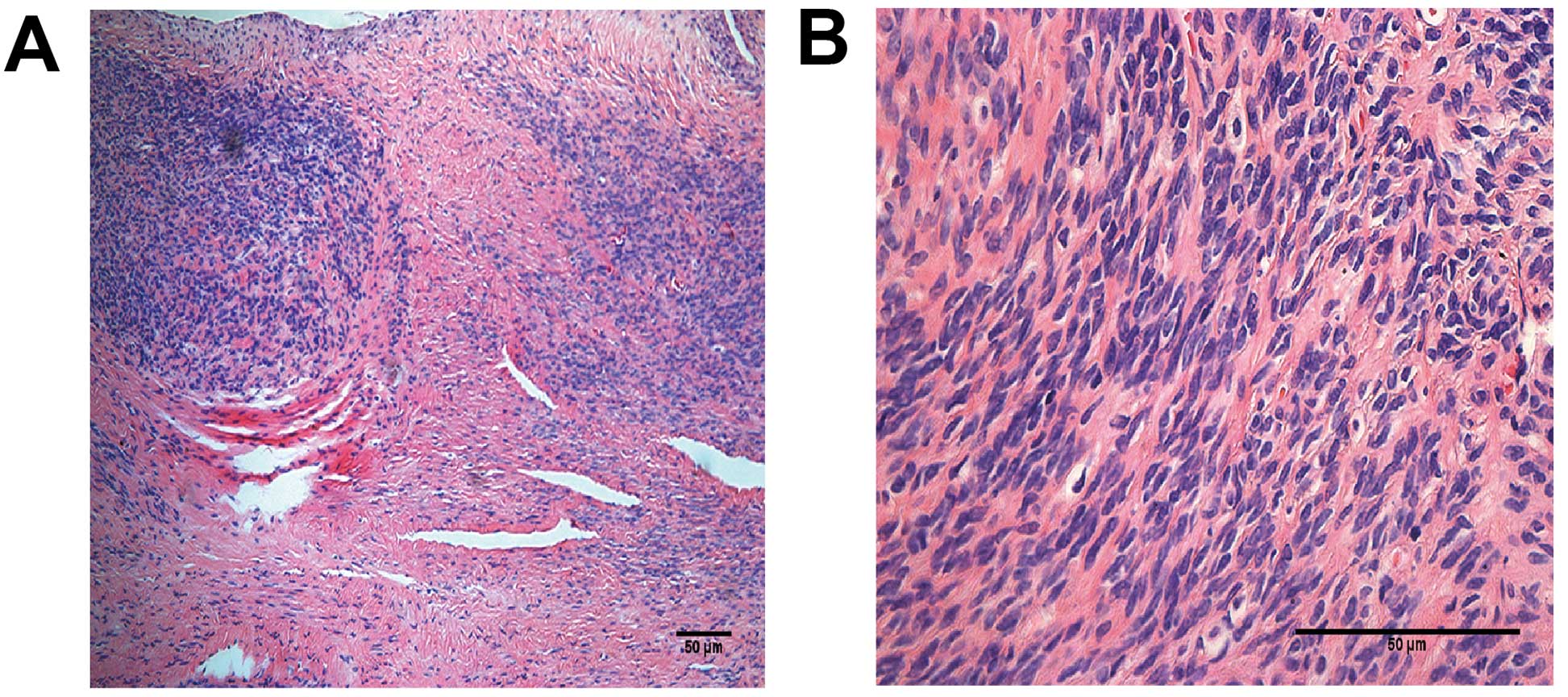
length; and the right ovary was sized 3×2.5×1.5 cm (Fig. 3). The postoperative pathological
examination indicated intravenous leiomyomatosis (Fig. 4). Follow-up 3D-cardiac
ultrasonography revealed no visible echo in the inferior vena cava
or the right atrium (Fig. 2D).
Discussion
The etiology of intravenous leiomyomatosis remains
unclear, but two theories have been proposed: One suggests that
intravenous leiomyomatosis originates from smooth muscle cells in
the vessel wall, whereas the other suggests that intravenous
leiomyomatosis arises from a uterine leiomyoma, with the benign
tumor cells invading the uterine veins and continuing to grow along
the venous circulation (2,3).
Ordulu et al (4) attempted to explain the pathogenesis of
intravenous leiomyomatosis by molecular cytogenetic analyses, and
they suggested that dysregulation of the non-histone
chromatin-associated architectural factor HMGA2, which affects the
differentiation and proliferation at 12q14, plays a role in the
development of intravenous leiomyomatosis. Leiomyomatosis
peritonealis disseminata (LPD) is a subtype of intravenous
leiomyomatosis that usually occurs in women of reproductive age.
Yuri et al (5) reported that
LPD lesions expressed progesterone receptor, while they were
negative for estrogen receptor and luteinizing hormone receptor
expression. Kokawa et al (6)
indicated that high levels of estradiol were associated with the
development of intravenous leiomyomatosis.
Intravenous leiomyomatosis extending to the atrium
may be confused with intracardiac tumors, such as myxoma and
lipoma, or thrombus formation, and cause multiple symptoms, such as
chest pain, breathlessness and syncope. Computed tomography (CT)
images may help identify lesions in the inferior vena cava.
However, as a proportion of the patients are reportedly
asymptomatic, it is crucial to make an early accurate diagnosis and
select the appropriate treatment schedule.
The majority of the patients have a history of
uterine leiomyoma or hysterectomy. Imaging is also important for
correct diagnosis. Gui et al (7) reported that CT angiography may reveal
the location, size and full-scale extension pathway of intravenous
leiomyomatous lesions, and maybe used as the first-line imaging
modality in preoperative assessment. When leiomyomatosis affects
the spine, magnetic resonance imaging may provide information for
the diagnosis and the extent of the lesions (8). Echocardiography with good penetration
of the tumor is also helpful in reaching a diagnosis (9).
There are currently no established guidelines
regarding the treatment of intravenous leiomyomatosis. However,
therapy must be individualized according to the patients' age,
hormonal and reproductive status and symptomatology. Surgery is the
only effective treatment for intravenous leiomyomatosis extending
to the inferior vena cava and the cardiac chambers. Surgical
treatment includes one-stage or two-stage surgery.
In the present case, we applied a series of
successful one-stage surgeries; the pelvic and chest surgeries were
performed at the same time. The type of surgery performed should be
also based on the patients' general condition and the size of the
tumor. Most researchers use cardiopulmonary bypass when excising
the mass in the inferior vena cava or the right atrium; however,
this is associated with an increased risk of ischemia and perfusion
injury, oxidative stress injury of vital organs (e.g., acute lung
injury and kidney injury) and thrombogenesis (10,11). In
the present case, we performed the surgery under non-extracorporeal
circulation, which may decrease non-physiological alterations and
postoperative complications, but may also increase the degree of
difficulty of the operation. Venous return was controlled by the
bilateral pinch-off method over a short time period (mean, 3–4
min). This treatment may provide a reference for other clinicians,
as successfully performing this surgery under non-extracorporeal
circulation was proven to be feasible.
A total of 11 cycles of GnRHa was administered prior
to the operation in this case, although the efficacy of hormonal
therapy (GnRHa) was questionable. However, a previous study
reported that GnRHa therapy following surgery in LPD may prevent
the recurrence of new lesions (12).
Evidence of long-term efficacy of postoperative treatment in
intravenous leiomyomatosis is lacking, and further investigation is
required. Doyle et al (13)
reported that aromatase inhibitors are effective in preventing
tumor progression and recurrence in patients with incompletely
resected intravenous leiomyomatosis with cardiac extension.
Postoperative patient follow-up is required, as
recurrence is frequent. Hereditary leiomyomatosis and renal
carcinoma (HLRCC) syndrome is an autosomal dominant syndrome that
results from mutations in the fumarate hydratase gene (14). The fumarate hydratase gene is located
on a highly conserved region of the 1q42.3–43 chromosome (15). Patients with HLRCC are commonly aged
10–44 years (although genetic testing should be offered to children
as young as 8–10 years of age) and at risk of uterine smooth muscle
tumors, as well as renal tumors (16). Therefore, it is imperative to
investigate patient history, and follow-up should include
evaluation of recurrence of the primary disease and occurrence of
correlative renal tumors.
One-stage resection was successfully completed in
this case under non-extracorporeal circulation. The diagnosis of
intravenous leiomyomatosis requires imaging combined with clinical
manifestations and history. Surgery is the standard treatment once
the diagnosis is established. Tissue biopsy is a definitive method
for diagnosing intravenous leiomyomatosis. The follow-up should be
started immediately after the hysterectomy in order to timely
detect early-stage intravenous leiomyomatosis.
Acknowledgements
The authors appreciate the assistance of Dr Zhang
Xiaoming and Dr Wei Lihui (Peking University People Hospital) with
directing the surgery. The present study was supported by grants
from the Shenzhen Municipal Science and Technical Innovation
Committee, Shenzhen Technical Research and Development Center on
Gynecologic Oncology (no. GCZX2015043016200372), the Science and
Technology Planning Project of Guangdong Province (no.
2013B021800095), and the Science and Technology Planning Project of
Shenzhen Municipal Government (no. JCYJ20140415162338852).
References
|
1
|
Mizuno T, Mihara A and Arai H:
Intracardiac and intravascular leiomyomatosis associated with a
pelvic arterio-venous fistula. Ann Transl Med. 2:482014.PubMed/NCBI
|
|
2
|
Kutay V, Tuncer M, Harman M, Ekim H and
Yakut C: Intracardiac extension of intravenous leiomyoma. Texas
heart institute journal/from the Texas heart institute of St.
Luke's episcopal hospital, Texas children's hospital. 32:232–234.
2005.
|
|
3
|
Nam MS, Jeon MJ, Kim YT, Kim JW, Park KH
and Hong YS: Pelvic leiomyomatosis with intracaval and intracardiac
extension: A case report and review of the literature. Gynecol
Oncol. 89:175–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ordulu Z, Nucci MR, Dal Cin P, Hollowell
ML, Otis CN, Hornick JL, Park PJ, Kim TM, Quade BJ and Morton CC:
Intravenous leiomyomatosis: An unusual intermediate between benign
and malignant uterine smooth muscle tumors. Mod Pathol. 29:500–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuri T, Kinoshita Y, Yuki M, Yoshizawa K,
Emoto Y and Tsubura A: Leiomyomatosis peritonealis disseminata
positive for progesterone receptor. Am J Case Rep. 16:300–304.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokawa K, Yamoto M, Yata C, Mabuchi Y and
Umesaki N: Postmenopausal intravenous leiomyomatosis with high
levels of estradiol and estrogen receptor. Obstet Gynecol.
100:1124–1126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gui T, Qian Q, Cao D, Yang J, Peng P and
Shen K: Computerized tomography angiography in preoperative
assessment of intravenous leiomyomatosis extending to inferior vena
cava and heart. BMC Cancer. 16:732015. View Article : Google Scholar
|
|
8
|
Hur JW, Lee S, Lee JB, Cho TH and Park JY:
What are MRI findings of Spine Benign Metastasizing Leiomyoma? Case
report with literature review. Eur Spine J. 24:(Suppl 4).
S600–S605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Shen Y, Sun Y, Zhang C, Yang Y, Yang
J, Su R and Jiang B: Intravenous leiomyomatosis with intracardiac
extension: Echocardiographic study and literature review. Tex Heart
Inst J. 41:502–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang FY, Bao YZ, Liu FS, Zhu YC, Zheng J,
Zhang JH, Zheng XF and Wei GC: Non-extracorporeal circulation for
coronary artery bypass graft surgery is more beneficial than
extracorporeal circulation. Eur Rev Med Pharmacol Sci.
19:1452–1456. 2015.PubMed/NCBI
|
|
11
|
Zhang YH, Jin CZ, Jang JH and Wang Y:
Molecular mechanisms of neuronal nitric oxide synthase in cardiac
function and pathophysiology. J Physiol. 592:3189–3200. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bisceglia M, Galliani CA, Pizzolitto S,
Ben-Dor D, Giannatempo G, Bergoli AL and Aieta M: Selected case
from the Arkadi M. Rywlin international pathology slide series:
Leiomyomatosis peritonealis disseminata: Report of 3 cases with
extensive review of the literature. Adv Anat Pathol. 21:201–215.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doyle MP, Li A, Villanueva CI, Peeceeyen
SC, Cooper MG, Hanel KC, Fermanis GG and Robertson G: Treatment of
intravenous leiomyomatosis with cardiac extension following
incomplete resection. Int J Vasc Med. 2015:7561412015.PubMed/NCBI
|
|
14
|
Joseph NM, Solomon DA, Frizzell N, Rabban
JT, Zaloudek C and Garg K: Morphology and immunohistochemistry for
2SC and FH aid in detection of fumarate hydratase gene aberrations
in uterine leiomyomas from young patients. Am J Surg Pathol.
39:1529–1539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bayley JP, Launonen V and Tomlinson IP:
The FH mutation database: An online database of fumarate hydratase
mutations involved in the MCUL (HLRCC) tumor syndrome and
congenital fumarase deficiency. BMC Med Genet. 9:202008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mann ML, Ezzati M, Tarnawa ED and Carr BR:
Fumarate hydratase mutation in a young woman with uterine
leiomyomas and a family history of renal cell cancer. Obstet
Gynecol. 126:90–92. 2015. View Article : Google Scholar : PubMed/NCBI
|