Introduction
Primary squamous cell carcinoma (SCC) of the thyroid
gland is rare, accounting for <1% of all thyroid cancers
(1). Primary SCC is often diagnosed
at a locally advanced stage (2) and
has a poor prognosis, with a median overall survival (mOS) of <6
months, which is clinically similar to undifferentiated carcinoma
of the thyroid gland with an aggressive course (3). While 43% of undifferentiated carcinomas
of the thyroid gland give rise to distant metastases (4), SCC of the thyroid gland has been
reported to metastasize in ~25% of the cases, whereas local
invasion is more frequent (5).
Thyroid cancer often metastasizes to the lung and
the bone, whereas metastasis to the heart is extremely rare, having
been identified in 0–2% of autopsy cases with thyroid cancer
(6). Since the diagnosis of cardiac
metastasis of thyroid cancer is difficult due to its asymptomatic
nature, only 54 cases have been diagnosed during their lifetime
with cardiac metastasis of thyroid cancer over a period of 130
years (7). Although the major
histological types of thyroid cancer have been reported to include
papillary, follicular and undifferentiated cancer, to the best of
our knowledge, no case of SCC of the thyroid gland with cardiac
metastasis has been reported to date.
Metastasis of thyroid cancer to the heart may cause
various specific symptoms and it has been associated with a poor
prognosis. While previous cases mainly resulted in sudden death due
to severe arrhythmia and heart failure (7), there has been one report of a
coagulation disorder associated with cardiac metastasis of
undifferentiated thyroid cancer (8).
The patient presented herein developed a severe
coagulation disorder in association with SCC of the thyroid gland
with cardiac metastasis. The coagulation disorder was controlled
using chemoradiotherapy for the metastatic lesion of the heart.
Case report
In April, 2015, a 57-year-old man became aware of a
neck mass and consulted the Department of Otolaryngology of the
Kyushu University Hospital (Fukuoka, Japan). The patient had no
significant past medical history, was a social drinker, had never
smoked and had no known allergies. There was no family history of
malignant tumors. A computed tomography (CT) scan revealed a
thyroid mass invading the trachea and enlargement of the cervical
lymph nodes. A fluorodeoxyglucose-positron emission tomography
(FDG-PET)/CT scan revealed high-uptake lesions in the thyroid gland
and cervical lymph nodes. Fine-needle aspiration biopsy of a
cervical lymph node revealed infiltration by papillary thyroid
carcinoma (PTC). The patient was diagnosed with PTC, T4aN1bM0,
stage IVA according to the 7th edition of the Union for
International cancer Control TNM classification of malignant tumors
(http://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf)
and underwent total thyroidectomy with bilateral modified neck
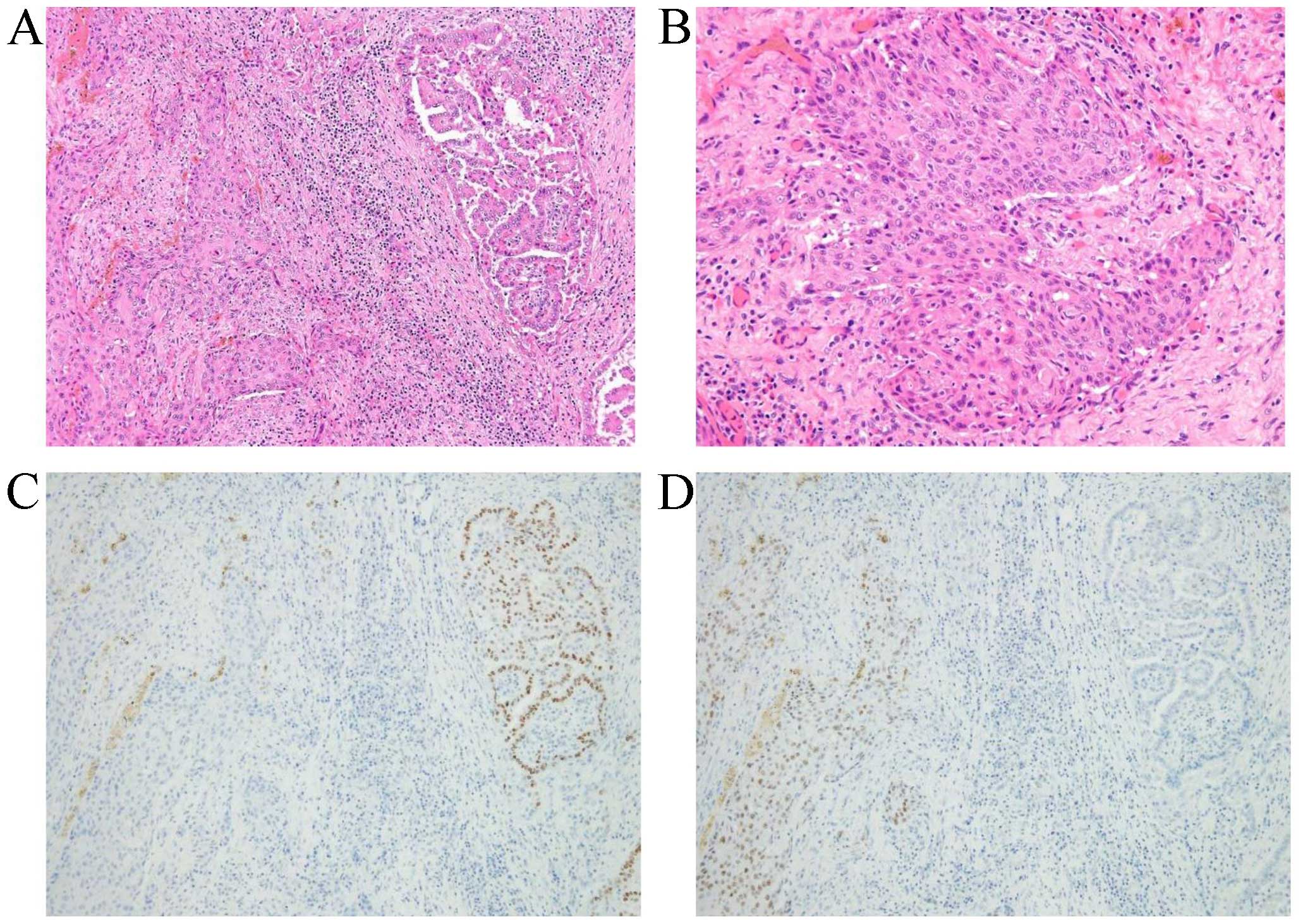
dissection and tracheotomy. On histological examination, the tumor
exhibited papillary carcinoma as well as squamous cell carcinoma
(SCC) components (Fig. 1). No
anaplastic carcinoma component was evident, which was consistent
with SCC of the thyroid gland transformed from PTC. The surgical
margin was positive for carcinoma cells, and 8 of the 30 retrieved
cervical lymph nodes were positive for metastases; thus, the
disease was histologically confirmed as pT4aN1bM0, stage IVA. Due
to the positive margin, the patient was administered postoperative
chemoradiotherapy consisting of an oral 5-fluorouracil derivative,
S-1 (80 mg/day), and radiation (61.4 Gy/33 fractions) as adjuvant
therapy.
Three months after the adjuvant therapy, the patient
returned complaining of subcutaneous hemorrhages of the
extremities. On admission, the blood pressure was 144/81 mmHg, the
heart rate was 115/min, the temperature was 36.8°C and the
SpO2 was 96%. The Eastern Cooperative Oncology Group
performance status was 2. Physical examination showed subcutaneous
hematomas involving the left thigh and lower leg. There was no
thrombocytopenia, but elevation of fibrin/fibrinogen degradation
products (FDP) (332.3 µg/ml) and D-dimer (35.1 µg/ml), and
consumption of fibrinogen (68 mg/dl) were confirmed on blood
examination. The laboratory evaluation was consistent with
disseminated intravascular coagulation (DIC). The levels of certain
tumor markers, such as carbohydrate antigen 19-9 (252.5 U/ml),
cytokeratin 19 fragment (10.8 ng/ml) and SCC antigen (2.4 ng/ml)
were elevated, but those of carcinoembryonic antigen (1.7 ng/ml)
and thyroglobulin (0.46 ng/ml) were within the normal range. The
patient was put on low-molecular-weight heparin therapy (75 IU/kg)
and replacement with fresh-frozen plasma. The CT scan revealed no
significant findings, apart from the hematomas of the left thigh
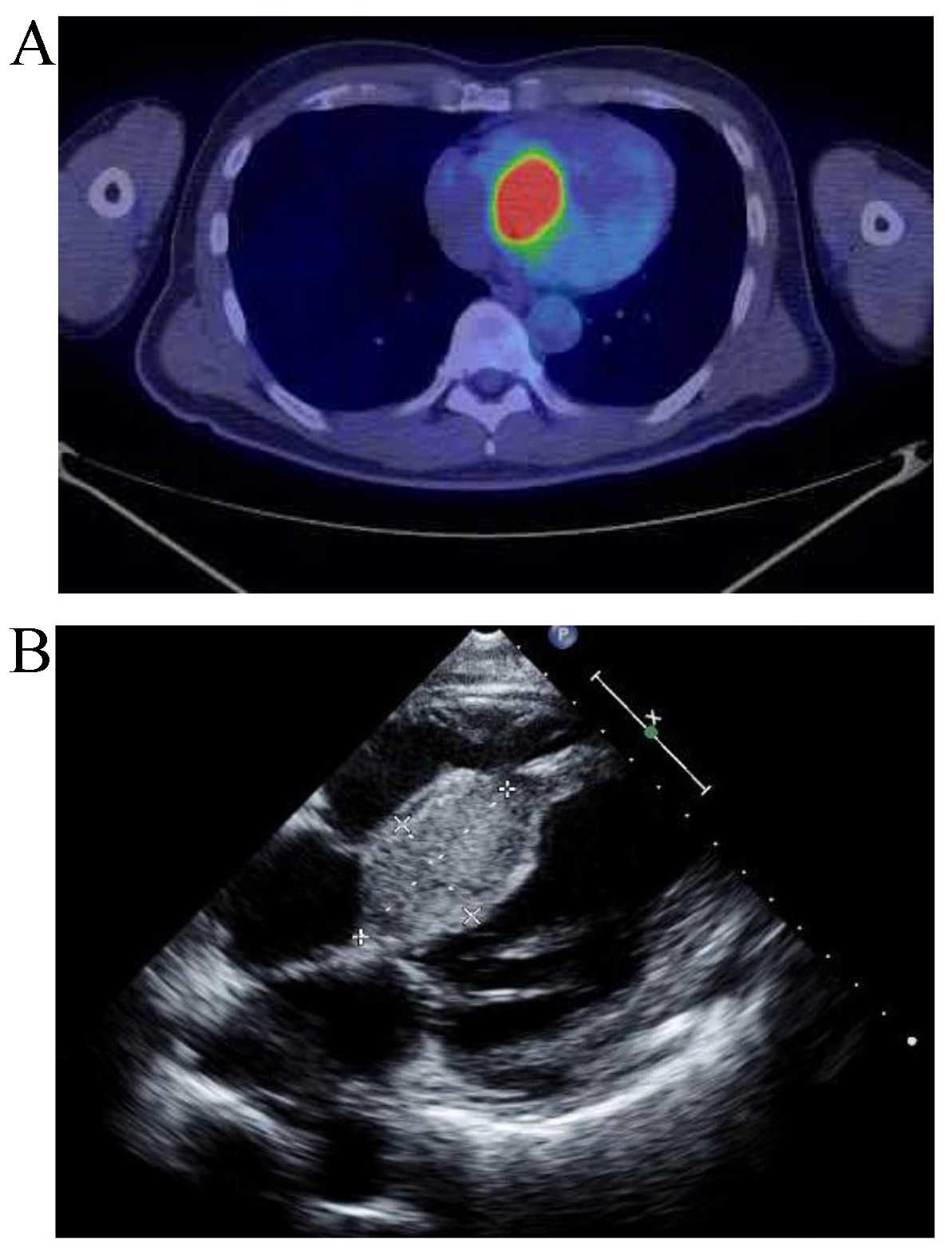
and lower leg. However, an FDG-PET scan revealed high-uptake
lesions in the interventricular septum and left hilar lymph nodes
(Fig. 2A). The patient was diagnosed
with cardiac and mediastinal lymph node metastases of SCC of the
thyroid gland with DIC. Echocardiography showed preserved systolic
function (69.9%) and a pulmonary arterial wedge pressure of 13.0
mmHg. No thrombosis was observed in the cardiac chambers, but a
mass sized 52 mm in greatest diameter was observed protruding
towards the right ventricle from the interventricular septum
(Fig. 2B). One month after
admission, the patient underwent placement of a cardiac pacemaker
for the management of complete atrioventricular block caused by
cardiac metastasis.
Following pacemaker placement, radiotherapy (41.4
Gy/23 fractions) concurrent with weekly administration of
paclitaxel (30 mg/m2/week, every 3 weeks) was initiated
for the treatment of cardiac and mediastinal lymph node metastases.
DIC resolved 18 days after the initiation of chemoradiotherapy, and
subcutaneous hemorrhage and leg pain improved significantly. Blood
tests showed a normal blood platelet count of
22.3×104/µl, fibrinogen of 223 mg/dl, mildly elevated
FDP of 10.6 µg/ml and D-dimer of 8.2 µg/ml. Although the
coagulation disorder was effectively controlled, the size of the
cardiac metastasis remained stable.
Approximately 2 weeks after the initiation of
chemoradiotherapy, cough and shortness of breath appeared, without
fever, respiratory failure, or sputum. The results of the blood
tests were as follows: White blood cell count 8,930/µl, CRP 10.01
mg/dl, Krebs von den Lungen-6 300 U/ml, surfactant protein-D 53.3
ng/ml and procalcitonin 0.28 ng/ml. The results of sputum and blood
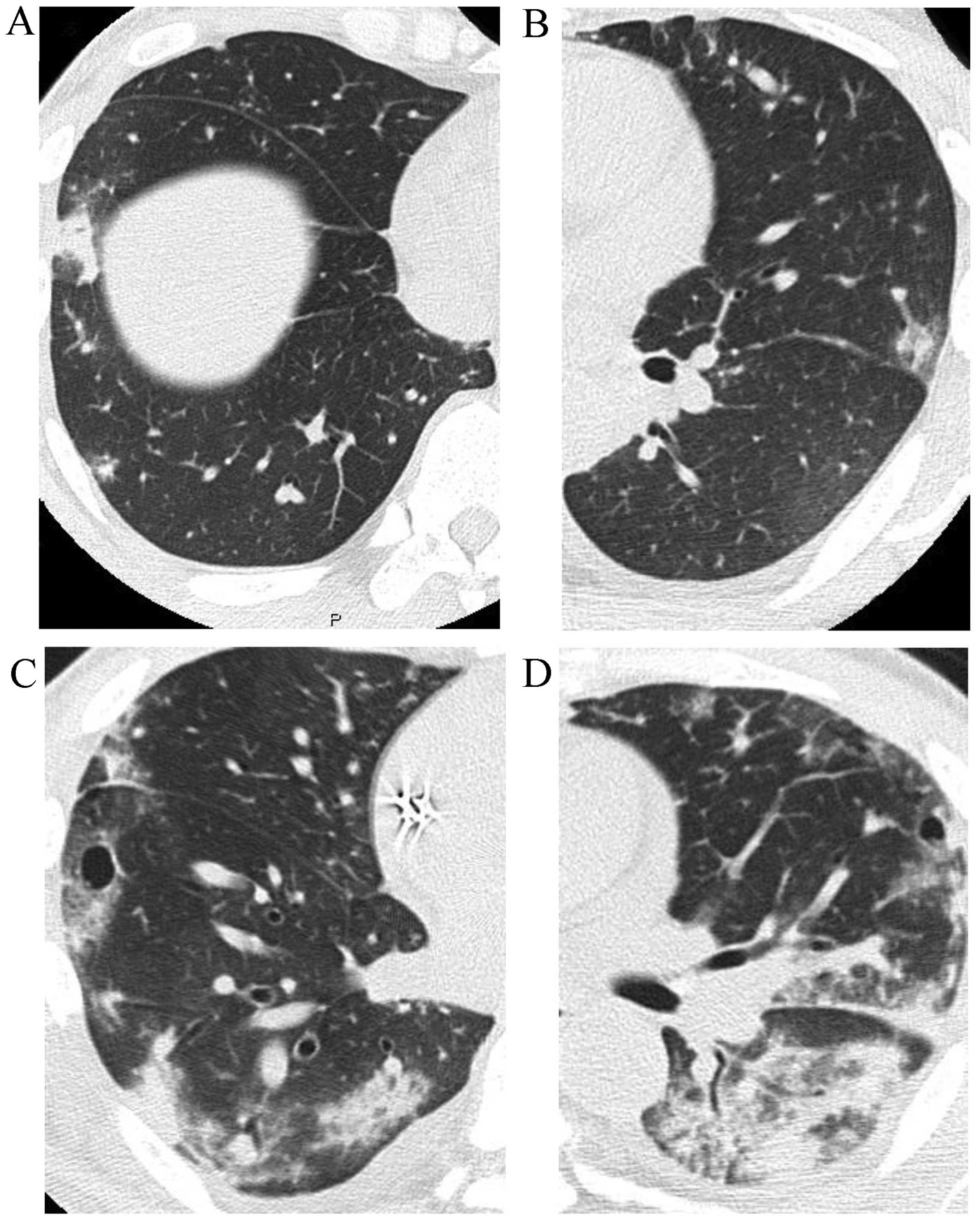
cultures were negative. CT revealed increased 1-cm nodular shadows
spreading in the subpleura of the lungs bilaterally and a pattern
of organizing pneumonia (Fig. 3A and
B), suggesting the effect of radiation. The bronchoalveolar
lavage fluid included 83% macrophages, 13% neutrophils and 4%
lymphocytes. Although the increased neutrophil fraction suggested
bacterial pneumonia, the CT findings supported radiation
pneumonitis and chemoradiotherapy was discontinued at 27 Gy/15
fractions. Prednisolone (0.5 mg/kg) was administered with a
combination of antibiotics. Despite treatment, respiratory failure
progressed and the lung field shadows gradually worsened. Since DIC
relapsed following cessation of chemoradiotherapy, systemic therapy
for the primary disease was deemed necessary. Lenvatinib (24
mg/day) was administered, but no response was observed on
echocardiography. Eighteen days after the administration of
lenvatinib, the patient's respiratory condition suddenly
deteriorated and the nodular shadows expanded to include a wide
area of the subpleura (Fig. 3C and
D). The DIC progressed further and rapid enlargement of the
protruding right ventricular mass was also observed on
echocardiography. Twenty-five days after administration of
lenvatinib, the patient succumbed to respiratory failure due to
alveolar hemorrhage induced by DIC.
Discussion
Thyroid cancer commonly metastasizes to the lung and
bone, while metastasis to the heart is extremely rare. Although
cardiac metastasis has been identified in 0–2% of autopsy cases of
thyroid cancer (6), the diagnosis of
this type of metastasis during the patient's lifetime is unusual
(7). A systematic review of rare
metastases of differentiated thyroid cancer, including 42 case
series and 197 case reports, demonstrated metastases to the brain,
skin and liver, but not to the heart (9). SCC of the thyroid gland often
progresses rapidly, similar to undifferentiated carcinoma of the
thyroid gland, but distant metastases are observed less frequently
compared with undifferentiated carcinoma. To the best of our
knowledge, the present study is the first report of SCC of the
thyroid gland with cardiac metastasis.
Carcinoma of the thyroid gland metastasizing to the
right and left ventricles and other parts of the heart has been
reported (7,8), with cardiac symptoms including
arrhythmia, heart failure and valvular disease. Since surgical
intervention for the tumor may achieve a good prognosis, it should
be considered; however, there are few such cases. The prognosis of
cardiac metastasis has not been fully elucidated, but is expected
to be poor based on the frequent cases of sudden death from heart
complications (7). Our patient also
suffered from impaired atrioventricular conduction, requiring
insertion of a pacemaker. Although the tumor was located in the
interventricular septum and protruded into the right ventricle,
there was no obvious right heart failure.
Severe DIC in a patient with a solid malignant tumor
is relatively rare. A prospective study by Sallah et al
demonstrated that 76 of 1,117 patients (6.8%) with solid tumors had
DIC (10). A multivariate analysis
in that study revealed that old age, breast cancer and necrosis in
the tumor tissue were risk factors for DIC (10). Additionally, high tumor burden with
multiple metastases and bone marrow metastasis of gastric or breast
cancer are often associated with DIC. Although a case of DIC in
association with cardiac metastasis from thyroid cancer was
previously reported, this finding is quite rare (8).
DIC in patients with solid tumors is mainly caused
by tissue factor production by the tumor cells and increasing
tissue factor accumulation on the surface of monocytes and
macrophages. Activation of the extrinsic pathway by excessive
tissue factors induces DIC, and direct exposure of the tumor
surface to the plasma may also contribute to induction of DIC
(11). A metastatic cardiac tumor
protruding into the ventricle may cause severe DIC, as reported in
a previous such case (12). The
metastatic tumor in the right ventricle is also considered to have
been the cause of the severe DIC in the present case.
Control of tumor-associated DIC requires not only
supportive care, but also antitumor therapy. A retrospective
analysis of the treatment of DIC in association with advanced
gastric or colorectal cancer demonstrated that the patient group
treated with chemotherapy was superior in terms of overall survival
and amelioration of symptoms caused by DIC to the group receiving
best supportive care (13). Although
one of the standard treatments for metastatic thyroid cancer is
chemotherapy with kinase inhibitors (14–16), it
was not possible in the present case due to the clinical bleeding
tendency. Weekly administration of paclitaxel (30 mg/m2)
was applied based on its survival benefit and safety profile
(17) for anaplastic thyroid cancer
and consideration of its safety with concomitant external beam
radiation (18). Intensive
supportive care did not improve DIC in our patient, but irradiation
of the metastases to the heart and the hilar lymph nodes in
combination with systemic chemotherapy with paclitaxel resulted in
significant improvement.
Lung metastasis appears in 7–12% of thyroid cancer
patients (19). Multiple nodular or
granular shadows sized 0.5–3 cm are generally observed on
radiographic examination (19).
Although no lung metastases were detected at the time of diagnosis
of the cardiac metastasis by CT and PET examinations in the present
case, diffuse irregular infiltrative shadows, sized 1 cm, appeared
in both peripheral lung fields after the initiation of
chemoradiotherapy. Bacterial, viral, or fungal infections were not
considered likely based on the symptoms and the results of the
cultures and laboratory tests. Steroid treatment was administered
for possible irradiation pneumonia, but was ineffective. Thyroid
cancer has been recognized as one of the diseases commonly
associated with pulmonary tumor embolism (20–22). Luo
et al reported pulmonary tumor embolism in a patient with
right ventricular metastasis of thyroid cancer based on the facts
that the patient had no deep venous thrombosis and was on
anticoagulant therapy (23).
Although histological diagnosis was not available, the presence of
a tumor protruding into the right ventricle and worsening of the
condition even with anticoagulant therapy suggested pulmonary tumor
embolism leading to acute respiratory distress syndrome in our
patient.
The aim of this study was to present an extremely
rare case of cardiac metastasis of SCC of the thyroid gland
complicated by a severe coagulation disorder. Intensive local
control of the metastatic tumor by concurrent chemoradiotherapy is
required to control the coagulation disorder in such cases and, in
case of unexplained DIC, cardiac metastasis should be
considered.
References
|
1
|
Zhou XH: Primary squamous cell carcinoma
of the thyroid. Eur J Surg Oncol. 28:42–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Syed MI, Stewart M, Syed S, Dahill S,
Adams C, McLellan DR and Clark LJ: Squamous cell carcinoma of the
thyroid gland: Primary or secondary disease? J Laryngol Otol.
125:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam KY, Lo CY and Liu MC: Primary squamous
cell carcinoma of the thyroid gland: An entity with aggressive
clinical behaviour and distinctive cytokeratin expression profiles.
Histopathology. 39:279–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YX, Zhang B, Wu YH, Liu WS, Liu SY,
Gao L, Xu ZG and Tang PZ: Primary squamous cell carcinoma of the
thyroid: Retrospective analysis of 28 cases. Zhonghua Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 48:143–147. 2013.(In Chinese). PubMed/NCBI
|
|
6
|
Abraham KP, Reddy V and Gattuso P:
Neoplasms metastatic to the heart: Review of 3314 consecutive
autopsies. Am J Cardiovasc Pathol. 3:195–198. 1990.PubMed/NCBI
|
|
7
|
Catford SR, Lee KT, Pace MD, Marasco SF,
Longano A and Topliss DJ: Cardiac metastasis from thyroid
carcinoma. Thyroid. 21:855–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara K, Ohno M, Takenaga M, Tsuneyoshi H,
Takeuchi H, Kashida M, Yamaguchi T, Machii K, Furuta S and Tohda E:
Metastatic thyroid cancer to the right ventricle causing
obstruction of the right ventricular outflow tract and associated
with disseminated intravascular coagulopathy: A case report. J
Cardiogr. 16:765–767. 1986.(In Japanese). PubMed/NCBI
|
|
9
|
Madani A, Jozaghi Y, Tabah R, How J and
Mitmaker E: Rare metastases of well-differentiated thyroid cancers:
A systematic review. Ann Surg Oncol. 22:460–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sallah S, Wan JY, Nguyen NP, Hanrahan LR
and Sigounas G: Disseminated intravascular coagulation in solid
tumors: Clinical and pathologic study. Thromb Haemost. 86:823–833.
2001.
|
|
11
|
Sutherland DE, Weitz IC and Liebman HA:
Thromboembolic complications of cancer: Epidemiology, pathogenesis,
diagnosis, and treatment. Am J Hematol. 72:43–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
John T and Davis ID: Ventricular
metastasis resulting in disseminated intravascular coagulation.
World J Surg Oncol. 24:292005. View Article : Google Scholar
|
|
13
|
Feinstein DI: Disseminated intravascular
coagulation in patients with solid tumors. Oncology (Williston
Park). 29:96–102. 2015.PubMed/NCBI
|
|
14
|
Wells SA Jr, Robinson BG, Gagel RF, Dralle
H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR,
et al: Vandetanib in patients with locally advanced or metastatic
medullary thyroid cancer: A randomized, double-blind phase III
trial. J Clin Oncol. 30:134–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomized, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashiyama T, Ito Y, Hirokawa M,
Fukushima M, Uruno T, Miya A, Matsuzuka F and Miyauchi A: Induction
chemotherapy with weekly paclitaxel administration for anaplastic
thyroid carcinoma. Thyroid. 20:7–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Onoda N, Sugitani I, Higashiyama T, Hara
H, Ito K, Kammori M, Sugino K, Suzuki S, Toda K, Yoshida A and
Miyauchi A: Concept and design of a nationwide prospective
feasibility/efficacy/safety study of weekly paclitaxel for patients
with pathologically confirmed anaplastic thyroid cancer
(ATCCJ-PTX-P2). BMC Cancer. 15:4752015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fraser RS, Muller NL, Colman N, et al:
Pulmonary neoplasmsDiagnosis of disease of the chest. 4th.
Philadelphia: W.B. Saunders Company; pp. 1405–1407. 1999
|
|
20
|
Bassiri AG, Haghighi B, Doyle RL, Berry GJ
and Rizk NW: Pulmonary tumor embolism. Am J Respir Crit Care Med.
155:2089–2095. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan CK, Hutcheon MA, Hyland RH, Smith GJ,
Patterson BJ and Matthay RA: Pulmonary tumor embolism: A critical
review of clinical, imaging, and hemodynamic features. J Thorac
Imaging. 2:4–14. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roberts KE, Hamele-Bena D, Saqi A, Stein
CA and Cole RP: Pulmonary tumor embolism: A review of the
literature. Am J Med. 115:228–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo H, Tulpule S, Alam M, Patel R, Sen S
and Yousif A: A rare silent killer: Right atrial metastasis of
thyroid hürthle cell carcinoma. Case Rep Oncol. 8:233–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|