Introduction
As the standard treatment for rectal cancer, open or
laparoscopic total mesorectal excision (TME) with pre- or
post-operative chemoradiotherapy (CRT) has resulted in a decrease
in local recurrence (1,2). Although postoperative CRT for locally
advanced rectal cancer markedly improves local control compared
with surgery alone or surgery plus irradiation (3), preoperative CRT is considered to be the
optimal therapeutic regimen for locally advanced low rectal cancer
(LARC) due to its improved local control (4). Preoperative CRT may be associated with
less acute toxicity and greater rates of sphincter-saving
procedures, and may increase the probability of curative tumor
resection when compared with postoperative CRT (4). Furthermore, tumor down-staging by CRT
may lead to a complete clinical or pathological response (4). However, the postoperative complication
rate of TME may increase with preoperative irradiation.
Although laparoscopic surgery is considered to be
the best option available for the surgical treatment of rectal
cancer due to its rates of local recurrence and survival, similar
to those of open surgery, few reports in the literature have
addressed the effects of preoperative CRT on laparoscopic surgery
(5). The aim of the present study
was to determine whether preoperative CRT has any adverse effects
on laparoscopic surgery in patients with LARC.
Materials and methods
Ethical approval
The institutional ethics committee of Osaka Medical
College Hospital approved the present study. Informed consent for
the therapy was obtained from all the patients prior to
chemoradiotherapy, after they had received a detailed description
of the procedure and its likely complications.
Patients
This retrospective cohort study comprised 156
consecutive patients with histologically confirmed primary
adenocarcinoma of the lower rectum with a distal margin of <10
cm from the anal verge, who underwent laparoscopic or open surgical
treatment following preoperative CRT at Osaka Medical College
Hospital between July 2006 and December 2013. The indications for
laparoscopic surgery at our hospital included a maximal tumor size
not in excess of 10 cm, and no evidence of either synchronous
resectable liver metastasis or distant metastasis. Indications for
preoperative CRT included full-thickness rectal cancers (T3 or T4),
as staged by magnetic resonance imaging or multi-detector computed
tomography, no prior radiation therapy administered to the pelvis,
and no evidence of para-aortic lymph node metastasis. All patients
were treated with 5-fluorouracil-based chemoradiation, at a 4,000
centrigray (cGy) total dose of pelvic irradiation. A daily fraction
of 200 cGy was administered five times per week. Chemotherapy
consisted of oral tegafur/uracil and leucovorin calcium. The dose
of tegafur/uracil was 300 mg/m2, and that of leucovorin
calcium was 75 mg/day during the radiotherapy. The patients
subsequently underwent surgery 6–8 weeks after having completed the
CRT. Pathological staging of the cancers was performed according to
postoperative pathological reports using the Japanese
Classification of Colorectal Carcinoma, Second English Edition
(6). The characteristics of the 156
patients (107 men and 49 women) are shown in Table I. The median age of the patients was
62 years (range, 35–80), and the median tumor size was 4.0 cm
(range, 2–10 cm). The distance from the anal verge was 0–5.0 and
5.1–10.0 cm in 52.5 and 47.5% of the patients, respectively.
Clinical stage T3 tumors were present in 96.8% of the patients,
whereas 3.2% patients had T4 tumors.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Number of
patients |
|---|
| Age in years
(median/range) | 62 (35–80) |
| Gender |
|
| Male | 107 (68.6%) |
|
Female | 49
(31.4%) |
| Tumor size in cm
(median/range) | 4.0 (2–10) |
| Tumor histology |
|
| Tub1/tub2 | 151 (96.8%) |
| Poor/muc/sig | 5 (3.2%) |
| Depth of
invasion |
|
| T3 | 151 (96.8%) |
| T4 | 5 (3.2%) |
| Tumor location |
|
| 0–5.0
cma | 82 (52.5%) |
| 5.1–10.0
cma | 74 (47.5%) |
In the present study, long-term outcomes were
examined by comparing 77 of the 156 patients, who were followed for
>40 months (the CRT group), with 39 patients who had undergone
laparoscopic surgery without preoperative chemoradiotherapy for
LARC between January 2004 and November 2008, and who were followed
for >60 months (the surgery-alone group). Data on the
surgery-alone group of patients was obtained retrospectively. The
characteristics of the two groups are summarized in Table II. The mean age of the patients in
the CRT group was 62.05±10.23 years, and in the surgery-alone
group, it was 62.46±9.18 years (P=0.83). Of the patients, 49 in the
CRT group were men and 28 were women, whereas in the surgery-alone
group, 27 were men and 12 were women (P=0.64). The mean tumor size
was 4.02±0.14 cm in the CRT group and 4.02±0.20 cm (P=1.00) in the
surgery-alone group. The distance from the anal verge was 4.13±2.03
cm in the CRT group, and 5.61±2.45 cm (P<0.008) in the
surgery-alone group. Of the tumors in the CRT group, 26 were
well-differentiated tubular adenocarcinoma (tub1) and 49 were
moderately differentiated tubular adenocarcinoma (tub2), whereas
two were of other types. In the surgery-alone group, 22 of the
tumors were tub1, and 17 were tub2 (P=0.09). In the CRT group, 13
patients had stage II, 42 had stage IIIa, and 22 had stage IIIb
disease clinically, whereas in the surgery-alone group, 11 patients
had stage II, 20 had stage IIIa, and 8 had stage IIIb disease
clinically (P=0.32).
 | Table II.Comparison of patient characteristics
in the two groups. |
Table II.
Comparison of patient characteristics
in the two groups.
| Characteristics | Preoperative CRT
group (n=77) | Surgery-alone group
(n=39) | P-value |
|---|
| Age (years) | 62.05±10.23 | 62.46±9.18 | 0.83 |
| Gender
(male/female) | 72/27 | 27/12 | 0.64 |
| Tumor size (cm) | 4.02±0.14 |
4.02±0.20 | 1.00 |
| Tumor distance from
the anal verge (cm) | 4.13±2.03 |
5.61±2.45 | <0.01 |
| Tumor histology
(tub1/tub2/others) | 26/49/2 | 22/17/0 | 0.09 |
| cStage
(II/IIIa/IIIb) | 13/42/22 | 11/20/8 | 0.32 |
Surgical procedure
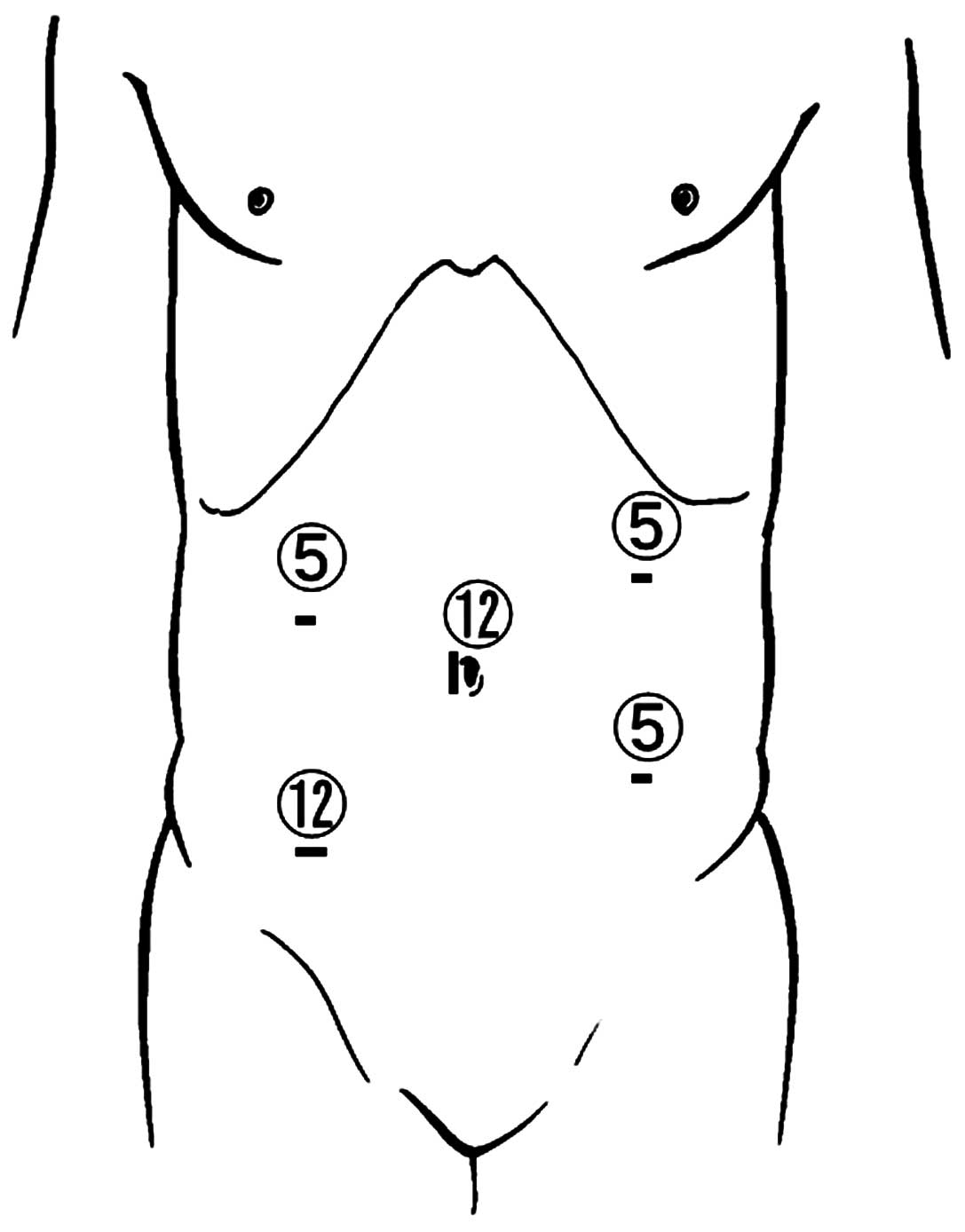
The five-port technique was used for laparoscopic
surgery, which featured: A 12-mm port at the navel; three 5-mm
ports, one each in the upper-right and -left and lower-left
abdominal quadrants; and a 12-mm port in the right lower quadrant,
as shown in Fig. 1. An Endopath™
Probe Plus II spatula probe (Ethicon Endo-Surgery, Cincinnati, OH,
USA) was used for precise dissection. After proximal lymph-node
dissection, TME of the rectum down to the floor of the pelvis was
performed. Subsequently, while preserving the hypogastric nerve,
dorsal dissection in the avascular plane between the mesorectum and
the parietal pelvic fascia down to the pelvic floor was precisely
and adequately performed. Care was taken not to damage the pelvic
splanchnic nerve during dorsal dissection. Next, lateral dissection
was completed, while ensuring that the hypogastric plexus was
preserved.
Dissection was performed up to the endopelvic fascia
and levator ani muscle, taking great care to preserve the
neurovascular bundle during the anterolateral dissection. Thus, in
principle, autonomic nerve-preserving surgery was performed, except
in the case of patients in whom we suspected direct tumor invasion
of the neural plexus. Echelon60™ (Ethicon Endo-Surgery) was used
for rectal resection in low or super-low (anastomosis within 2 cm
from the dentate line) laparoscopic anterior resection. The area of
the rectum contralateral to the planned dissection site was
retracted, and the tissue was pinched, as necessary, with the
stapler to accomplish rectal transection using a single-fire staple
cartridge. Stapling was performed from the anterior to the
posterior rectum wall. The specimen was extracted through the
diverting stoma incision, which was extended to ~3–4 cm, and the
anastomosis was intracorporeally completed using the
double-stapling technique. In cases of intersphincteric resection,
the specimen was extracted via the anus and a hand-sewn colo-anal
anastomosis was created. After abdominoperineal resection had been
performed, the specimen was retrieved in the usual manner through a
perineal incision. Primary perineal wound closure was performed,
and a terminal colostomy in the left lower quadrant site was
constructed. A well-experienced, board-certified laparoscopic
colorectal surgeon (J.O.) supervised all the surgical
operations.
Comparison between CRT and
surgery-alone groups
The 5-year relapse-free survival rates (RFS), local
pelvic recurrence free survival (LRFS) and 5-year overall survival
rates (OS) were determined in 77 of the CRT group patients who were
followed for >40 months, and the rates were compared with those
of the surgery-alone group.
Statistical analysis
Statistical analysis was performed using JMP 9 for
Windows (SAS Institute, Inc., Cary, NC, USA). Correlations between
categorical variables were assessed using the Chi-square test, and
continuous data were evaluated using the Mann-Whitney U-test.
Patient survival rates were calculated using the Kaplan-Meier
method, and survival curves were compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Surgical treatment and pathological
data
The surgical data are summarized in Table III. The rate for low or super-low
anterior resection was 64.7%; that for intersphincteric resection
was 15.4%, and that for abdominoperineal resection was 17.3%.
Diverting ileostomies were created in all patients, with the
exception of those who underwent abdominoperineal resection.
Sphincter-preserving surgery was performed in 74% of the patients,
and laparoscopic surgery was performed in 97.4% of the patients.
The histological data following surgery are summarized in Table IV. None of the patients had a
positive longitudinal resection margin, and only 1.3% of the
patients had a positive circumferential resection margin.
 | Table III.Surgical data. |
Table III.
Surgical data.
| Operative
procedure | Number of patients
(%) |
|---|
|
Laparoscopic surgery | 152 (97.4) |
| Low
anterior resection/super-low anterior resection | 101 (64.7) |
|
Intersphincteric
resection | 24
(15.4) |
|
Abdominoperineal
resection | 27
(17.3) |
| Open surgery | 4
(2.6) |
|
Abdominoperineal
resection | 3
(1.9) |
| Total
pelvic exenteration | 1
(0.6) |
 | Table IV.Histological data following
surgery. |
Table IV.
Histological data following
surgery.
| Type of surgery | Number of patients
(%) |
|---|
| Circumferential
resection margin |
|
|
Positive | 2 (1.3) |
|
Negative | 154 (98.7) |
| Distal resection
margin |
|
|
Positive | 0 (0) |
|
Negative | 156 (100) |
The results of histological assessment of the
responses to CRT are summarized in Table
V. We evaluated responses using the Japanese Classification of
Colorectal Carcinoma, Second English Edition (6). Accordingly, a grade 0 response (no
effect) indicated that tumor cell necrosis or degeneration was not
present in response to treatment. Grade 1a (minimal effect)
indicated the presence of tumor cell necrosis or degeneration in
less than one-third of the lesion. A grade 1b response (mild
effect) indicated the presence of tumor cell necrosis, degeneration
and/or lytic changes in more than one-third, but less than
two-thirds, of the lesion. A grade 2 response (moderate effect)
indicated that prominent tumor cell necrosis, lytic changes,
degeneration, and/or cancer disappearance occurred in more than
two-thirds of the lesion, although viable tumor cells still
remained. A grade 3 response (marked effect) indicated the presence
of necrosis and/or lytic changes throughout the lesion, which were
replaced by fibrosis with or without granulomatous changes, and no
viable tumor cells were observed. Our results revealed that a grade
0 response was not observed in any patients; a grade 1a response
was present in 31.4% of the patients; a grade 1b response was
present in 20.5% of the patients; a grade 2 response was in 29.5%
of the patients; and a grade 3 response was in 18.6% of the
patients. The resected specimen following surgery was submitted for
pathological evaluation. Microscopic positive circumferential
resection margins were present in only two cases, and microscopic
positive distal resection margin cases were not observed in any of
the cases (Table IV).
 | Table V.Histological assessments of response
to preoperative chemoradiotherapy. |
Table V.
Histological assessments of response
to preoperative chemoradiotherapy.
| Response to
NACRT | Number of patients
(%) |
|---|
| Grade |
|
| 0 | 0 (0) |
| 1a | 49 (31.4) |
| 1b | 32 (20.5) |
| 2 | 46 (29.5) |
| 3 | 29 (18.6) |
The surgical outcomes in the 156 patients who
underwent CRT are summarized in Table
VI. The total percentage of patients who experienced
complications was 14.1%; that of anastomotic leakage was 2.6%, and
that of wound infections was 5.8%. All the identified infections
were perineal wound infections following abdominoperineal
resection. Three patients had ileus, and two patients required
reoperation. Serious complications were not observed in the present
study, and no patients succumbed to mortality in the hospital.
 | Table VI.Postoperative mortality and
morbidity. |
Table VI.
Postoperative mortality and
morbidity.
| Characteristic | Number of patients
(%) |
|---|
| Postoperative
complication | 22 (14.1) |
|
Anastomotic leakage | 4 (2.6) |
| Wound
infection | 9 (5.8) |
|
Ileus | 3 (1.9) |
| Pelvic
abscess | 3 (1.9) |
| Urinary
disorder | 3 (1.9) |
| Mortality | 0 (0) |
OS, RFS and LRFS
The median follow-up period in the CRT group
patients was 58 months, and that in the surgery-alone group was 60
months. No significant differences were identified between the CRT
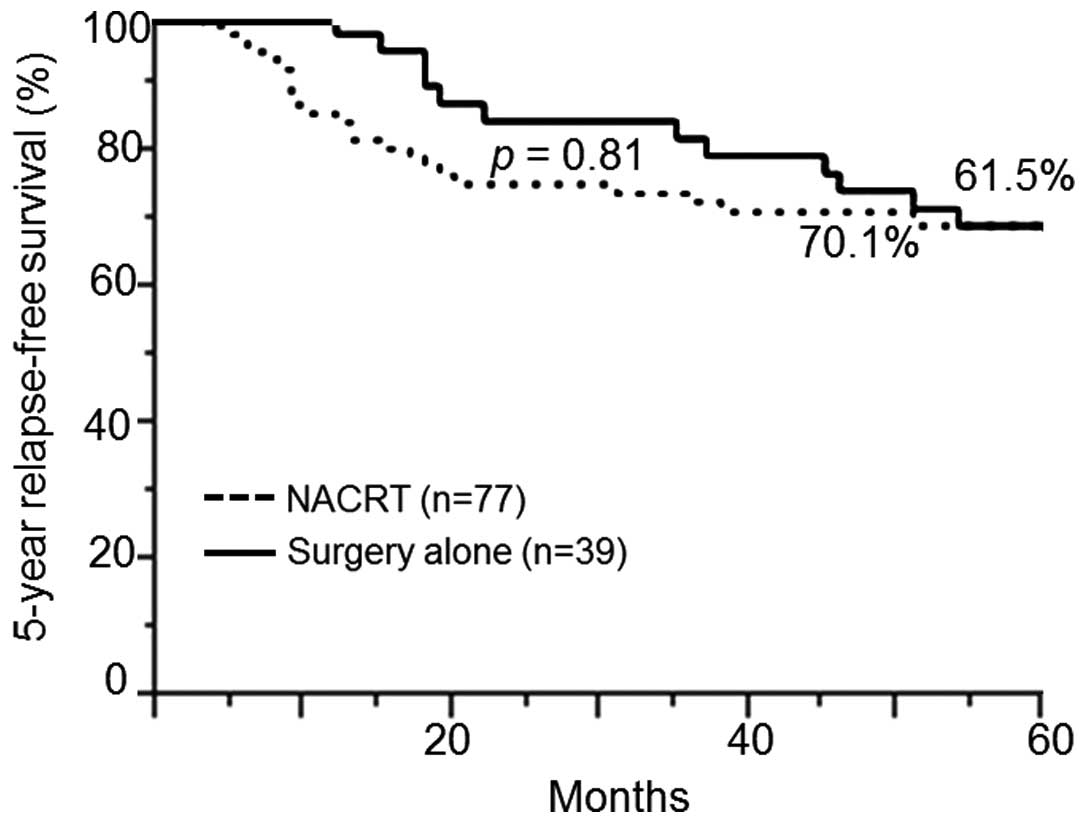
group and the surgery-alone group in terms of the 5-year RFS (70.1
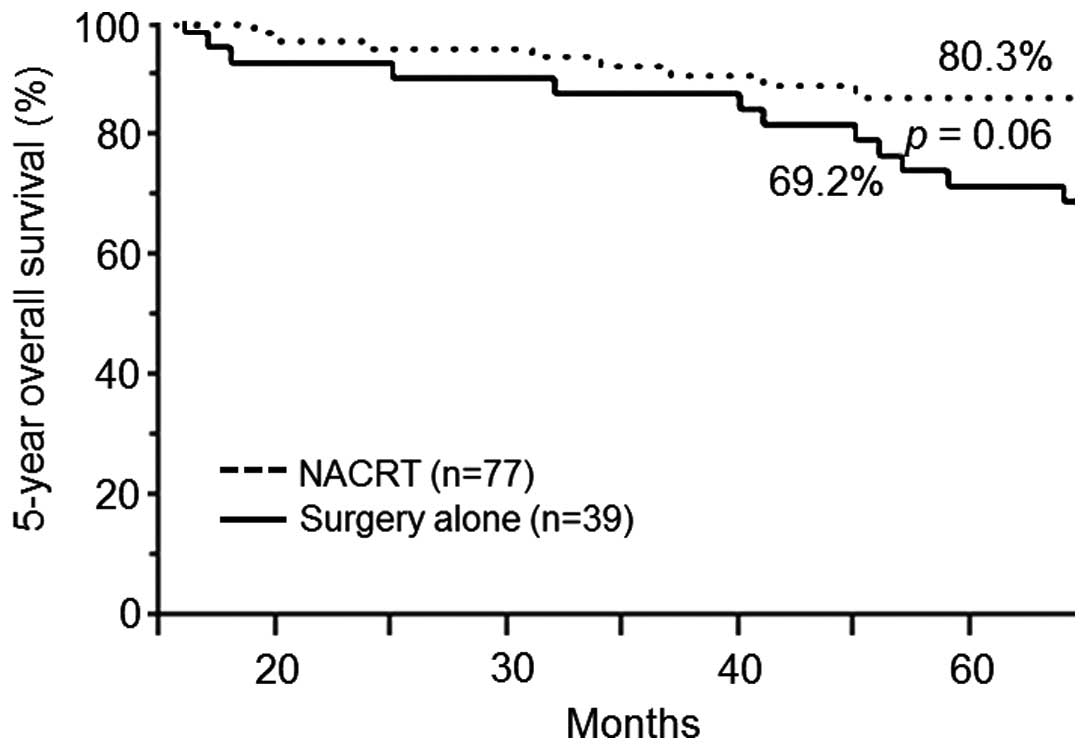
vs. 61.5%; P=0.81) (Fig. 2) and OS
(88.3 vs. 69.2%; P=0.06) (Fig. 3).
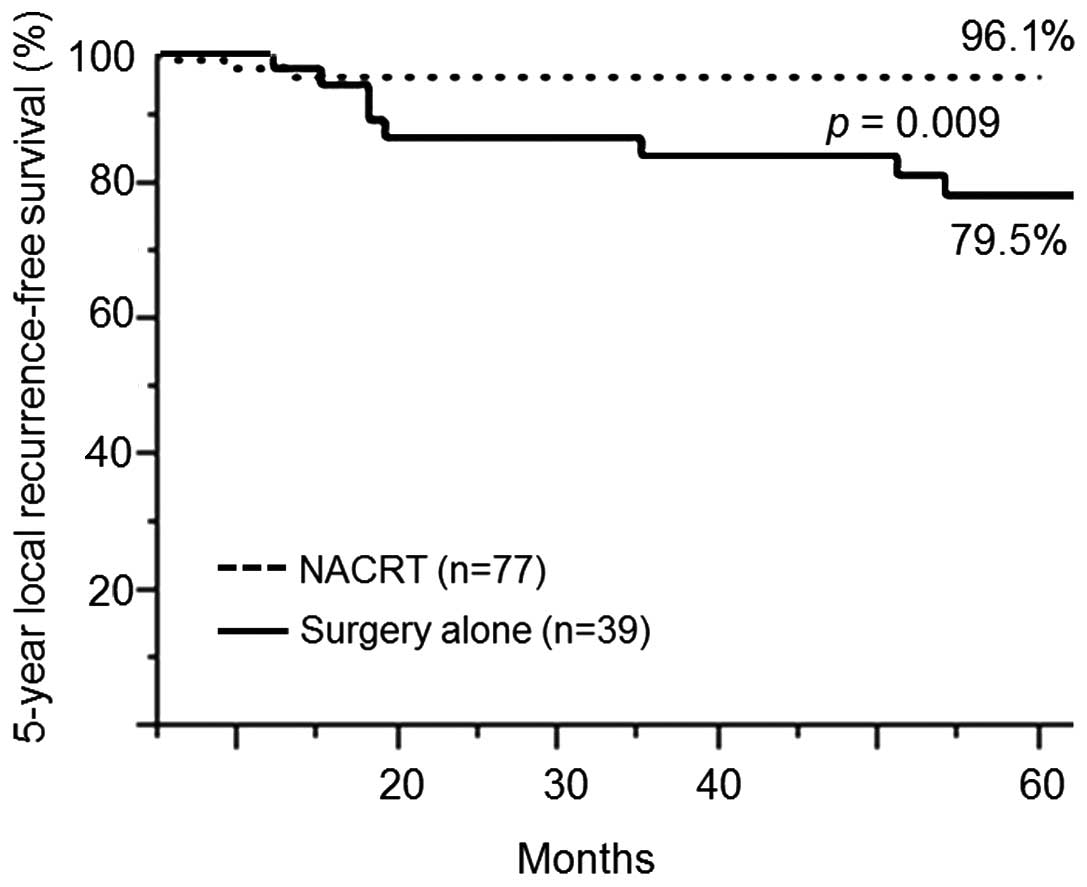
The CRT group had a significantly higher rate of LRFS at 5 years
compared with the surgery-alone group (96.1 vs. 79.5%; P=0.009)
(Fig. 4). The rates of recurrence
and/or metastasis following surgery are summarized in Table VII. Recurrence and/or metastasis
occurred in 29.9% of the patients in the CRT group. Of these, lung
metastasis accounted for 16.9%, liver metastasis for 2.6%, lymph
node metastasis for 3.9%, and local recurrence for 5.2%. of the
patients. By contrast, the recurrence and/or metastasis rate in the
surgery-alone group was 38.5%, with local recurrence rates of
20.5%, which was a higher percentage compared with that observed in
the CRT group. Lung metastasis occurred in 7.7%, and liver
metastasis in 2.6%, of the patients in the surgery-alone group. The
rate of local recurrence was higher in the surgery-alone group
compared with in the CRT group, whereas the CRT group had a higher
rate of lung metastasis.
 | Table VII.First recurrence and/or metastasis
following surgery. |
Table VII.
First recurrence and/or metastasis
following surgery.
| Characteristic | Preoperative CRT
group (n=77) (%) | Surgeryalone group
(n=39) (%) |
|---|
| Presence of
recurrence/metastasis | 23 (29.9) | 15 (38.5) |
| Local
recurrence | 4 (5.2) | 8 (20.5) |
| Lung
metastasis | 13 (16.9) | 3 (7.7) |
| Liver
metastasis | 2 (2.6) | 1 (2.6) |
| Lung
and liver metastasis | 1 (1.3) | 0 (0) |
| Lymph
node metastasis | 3 (3.9) | 1 (2.6) |
| Others | 0 (0) | 2 (5.1) |
Discussion
The present study has suggested that laparoscopic
surgery performed following preoperative CRT for advanced low
rectal cancer may be safely performed by skilled surgeons.
Laparoscopic resection of the colon is accepted as a method of
surgical treatment for colonic cancer (7,8).
However, the laparoscopic procedure for rectal cancer is
technically more difficult compared with that for colon cancer.
Although a previous study has suggested the safety and feasibility
of laparoscopic surgery for rectal cancer (9), its use continues to be controversial.
However, laparoscopic surgery for colorectal cancers has been used
to positive effect in our hospital (10–13) due
to its advantages in providing a good view, even in narrow
pelvises, and its more precise preservation of autonomic nerve
preservation (14). The difficulty
with surgery following CRT is partly explained by tissue
inflammation, and the edema that may occur following CRT.
Furthermore, although preoperative CRT in certain cases may blur
the dissection plane due to fibrosis, it was possible to
successfully perform nerve-preserving TME in all of our CRT
laparoscopic cases, thereby suggesting the safety and feasibility
of laparoscopic surgery following CRT.
The overall rate of postoperative complications in
the preoperative CRT group was 14.1%, of which only two patients
required reoperation. These two male patients had undergone
laparoscopic abdominoperineal resection (APR), and an ileus
occurred due to prolapse of the small intestine to the pelvic
floor. At present, since the current procedure is that the pelvic
peritoneum is repaired in male patients who undergo laparoscopic
APR, there have been no more cases of intestinal obstruction. A
previous study has reported much higher rates of anastomotic
leakage in patients undergoing laparoscopic resection for rectal
cancer (14) compared with those
reported in our study (four patients; 2.6%). Although
intracorporeal rectal transection and anastomosis require great
skill in patients undergoing laparoscopic low anterior resection,
our group has completely standardized this technique in the Osaka
Medical College Hospital, which has led to shorter operating times,
low blood loss and a very low rate of conversion (13).
A previous study reported preoperative irradiation
to be a risk factor for the development of surgical site infection
(14). The wound infections
occurring in the CRT group in our study included perineal wound
infections that followed abdominoperineal resection, suggesting
that a high rate of wound infection may not be a result of
preoperative CRT, but rather is associated with differences in
operative procedures among the different studies (15).
Through the use of several randomized controlled
trials (16), surgeons in western
countries have made preoperative CRT a standard therapeutic
approach for the treatment of LARC. Surgeons in Japan, however,
treat low rectal cancer with extended pelvic lymphadenectomy using
TME. Retrospective Japanese studies have shown that this procedure
decreases local recurrence while prolonging survival (17–19).
Recently, several studies have compared CRT, the standard in
western countries, with extended pelvic lymphadenectomy, the
standard in Japan (20,21). Despite a number of issues associated
with these studies, a great step towards the future improvement of
the treatment of low rectal cancer has been made by objectively
comparing these two standards.
Several studies have demonstrated that preoperative
CRT leads to an improvement in locoregional disease control,
although they did not demonstrate that preoperative CRT leads to
improved OS and RFS (1–4,16,22). The
results of the present study and those other recently performed
studies are, therefore, similar. Our hypothesis is that adjuvant
chemotherapy is likely to be of great importance in improving OS
and RFS rates in the future.
In conclusion, although the present data was
retrospectively collected, our results indicate that laparoscopic
surgery with preoperative CRT appears to be safe and feasible, and
may reduce local recurrence. However, future prospective studies
and randomized controlled trials are required to clarify the
benefits of preoperative CRT prior to laparoscopic surgery.
References
|
1
|
Kapiteijn E, Marijnen CA, Nagtegaal ID,
Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B,
van Krieken JH, et al: Preoperative radiotherapy combined with
total mesorectal excision for resectable rectal cancer. N Engl J
Med. 345:638–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and lecovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar
|
|
3
|
Krook JE, Moertel CG, Gunderson LL, Wieand
HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC and
Mailliard JA: Effective surgical adjuvant therapy for high-risk
rectal carcinoma. N Engl J Med. 324:709–715. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiation for rectal
cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiyoshi T, Kuroyanagi H, Oya M, Konishi
T, Fukuda M, Fujimoto Y, Ueno M, Yamaguchi T and Muto T: Safety of
laparoscopic total mesorectal excision for low rectal cancer with
preoperative chemoradiation therapy. J Gastrointest Surg.
13:521–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese classification of colorectal carcinoma. 2nd.
English edition. Kanehara & Co., Ltd.; Tokyo: 2009
|
|
7
|
Clinical Outcomes of Surgical Therapy
Study Group, . A comparison of laparoscopically assisted and open
colectomy for colon cancer. N Engl J Med. 350:2050–2059. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guillou PJ, Quirke P, Thorpe H, Walker J,
Jayne DG, Smith AM, Heath RM and Brown JM: MRC CLASICC trial group:
Short-term endpoints of conventional versus laparoscopic-assisted
surgery in patients with colorectal cancer (MRC CLASICC trial):
Multicentre, randomized controlled trial. Lancet. 365:1718–1726.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tjandra JJ, Chan MK and Yeh CH:
Laparoscopic- vs. hand-assisted ultralow anterior resection: A
prospective study. Dis Colon Rectum. 51:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto M, Okuda J, Tanaka K, Kondo K,
Tanigawa N and Uchiyama K: Clinical outcomes of laparoscopic
surgery for advanced transverse and descending colon cancer: A
single-center experience. Surg Endosc. 26:1566–1572. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi M, Komeda K, Inoue Y, Shimizu T,
Asakuma M, Hirokawa F, Okuda J, Tanaka K, Kondo K and Tanigawa N:
Simultaneous laparoscopic resection of colorectal cancer and
synchronous metastatic liver tumor. Int Surg. 96:74–81. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayano H, Okuda J, Tanaka K, Kondo K and
Tanigawa N: Evaluation of the learning curve in laparoscopic low
anterior resection for rectal cancer. Surg Endosc. 25:2972–2979.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuda J, Tanaka K, Kondo K, Asai K, Kayano
H, Yamamoto M and Tanigawa N: Safe anastomosis in laparoscopic low
anterior resection for rectal cancer. Asian J Endosc Surg. 4:68–72.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bärlehner E, Benhidjeb T, Anders S and
Schicke B: Laparoscopic resection for rectal cancer: Outcomes in
194 patients and review of the literature. Surg Endosc. 19:757–766.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konishi T, Watanabe T, Kishimoto J and
Nagawa H: Elective colon and rectal surgery differ in risk factors
for wound infection: Results of prospective surveillance. Ann Surg.
244:758–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koyama Y, Moriya Y and Hojo K: Effects of
extended systematic lymphadenectomy for adenocarcinoma of the
rectum-significant improvement of survival rate and decrease of
local recurrence. Jpn J Clin Oncol. 14:623–632. 1984.PubMed/NCBI
|
|
18
|
Moriya Y, Sugihara K, Akasu T and Fujita
S: Importance of extended lymphadenectomy with lateral node
dissection for advanced lower rectal cancer. World J Surg.
21:728–732. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugihara K, Moriya Y, Akasu T and Fujita
S: Pelvic autonomic nerve preservation for patients with rectal
carcinoma. Oncologic and functional outcome. Cancer. 78:1871–1880.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JC, Takahashi K, Yu CS, Kim HC, Kim
TW, Ryu MH, Kim JH and Mori T: Comparative outcomes between
chemoradiation therapy and lateral pelvic lymph node dissection
following total mesorectal excision in rectal cancer. Ann Surg.
246:754–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagawa H, Muto T, Sunouchi K, Higuchi Y,
Tsurita G, Watanabe T and Sawada T: Randomized, controlled trial of
lateral node dissection vs. nerve-preserving resection in patients
with rectal cancer after preoperative radiotherapy. Dis Colon
Rectum. 44:1274–1280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colorectal Cancer Collaborative Group:
Adjuvant radiotherapy for rectal cancer, . A systematic overview of
8,507 patients from 22 randomised trials. Lancet. 358:1291–1304.
2001.PubMed/NCBI
|