Introduction
The term ‘fever of unknown origin’ (FUO) was first
introduced by Petersdorf and Beeson in 1961 based on an analysis of
100 cases, and it was defined as recurrent fever >38.3°C,
lasting for >3 weeks, remaining undiagnosed after 1 week of
in-hospital evaluation (1). Several
decades later, the criteria of FUO diagnosis have changed and it is
currently defined by lack of a definitive diagnosis after
appropriate inpatient or outpatient evaluation (2). In view of the patient's clinical
circumstances and underlying immune status, FUO was categorized
into classic, nosocomial, neutropenic and human immunodeffiency
virus (HIV)-associated FUO by Durack and Street in 1991 (3). The etiologies of classic FUO mainly
include infections, malignancies, non-infectious inflammatory
diseases and miscellaneous causes, while some cases remain
undiagnosed (4,5).
Primary splenic lymphoma (PSL) is a rare malignant
lymphoma with an incidence of ~1% among patients with non-Hodgkin
lymphoma (NHL) (6), although the
spleen is involved in approximately half of the cases of Hodgkin's
disease and one-third of NHLs as part of systemic disease (7,8).
Dasgupta et al (9) strictly
defined PSL as lymphoma originating in the spleen and limited to
the spleen and splenic hilum, without invasion of other sites, with
an interval of ≥6 months prior to the appearance of lymphoma
elsewhere.
We herein present the case of a 59-year-old male
patient with diffuse large B-cell PSL, who only exhibited sustained
fever, without other remarkable complaints. To investigate the
cause of the FUO, fluorine-18-fluorodeoxyglucose positron emission
tomography/computed tomography (18F-FDG-PET/CT)
examination was performed and revealed a diffusely enlarged
hypermetabolic spleen. Following diagnostic splenectomy, the
histopathological diagnosis was diffuse large B-cell primary
splenic NHL.
Case report
A 59-year-old male patient presented with a history
of fever for 1 month. The temperature reached >39°C, with
episodes of chills, and the fever subsided spontaneously several
hours later, followed by sweating, which happened 1–2 times/day. A
mild cough with a small amount of white foamy phlegm were the only
other complaints. The patient's condition had been treated as an
infectious disease, with administration of cephalosporin for 7 days
and sequential quinolone for 2 days prior to hospitalization, but
without improvement of the symptoms. The patient had undergone
perianal abscess removal surgery 1 year prior, and denied a history
of tobacco, alcohol, or illicit drug use. There was also no report
of food or medication allergies.
On admission, the patient's temperature was 36.8°C,
with a pulse rate of 70 beats per minute. The findings of the
physical examination were unremarkable, apart from bilateral moist
crackles and coarse breath sounds in the lungs, dental caries and
multiple folliculitis on the face and neck. On laboratory
investigation, the complete blood count revealed mild anemia, with
a hemoglobin level of 116 g/l. Urinalysis revealed proteinuria and
occult blood, and the biochemical profile revealed mild hepatic
function test abnormalities, with a total protein level of 52 g/l,
an albumin level of 29 g/l and a serum lactate dehydrogenase level
of 626 U/l. The patient had increased parameters of inflammation
and infection, with an erythrocyte sedimentation rate (ESR) of 59
mm/h (normal, <20 mm/h), a high-sensitivity C-reactive protein
(CRP) level of 63.7 mg/l (normal, <8 mg/l) and a procalcitonin
level of 23.96 ng/ml (normal, <0.5 ng/l). The indicators of
autoimmune disease, such as extractable nuclear antigen and
antineutrophil cytoplasmic antibodies, were all negative. Screening
for specific infectious diseases, including tuberculosis, malaria,
enteric fever, viral infections such as HIV, cytomegalovirus,
Epstein-Barr virus (EBV) and coxsackievirus, was negative. The
blood, urine and stool cultures were negative. However, the sputum
culture was positive for Enterobacter cloacae and Candida
albicans. CT scanning of the chest revealed bilateral
interstitial changes in the lung bases, with enlarged lymph nodes
in the mediastinum. Abdominal ultasonography revealed splenomegaly
(13.2×4.5 cm) with normal echotexture. The findings of magnetic
resonance imaging of the brain were normal.
Taken together, the results of these examinations
indicated that infection occurred during the course of the disease,
but the infectious factor was clearly not the only cause of the
fever, as the patient's temperature would still increase to ≤40°C,
whether all antibiotics were stopped on admission, as the patient's
vital signs were stable, or whether empirical therapy was adopted
using antibiotics against gram-positive or -negative bacteria and
fungi, intermittent steroids, and even diagnostic treatment with
antimalarials.
To clearly determine the cause of the unexplained
fever, more specialized examinations were performed. The peripheral
blood and bone marrow aspiration films did not reveal any
abnormalities, and the bone marrow culture was negative. However,
the patient's condition did not improve and he developed dyspnea,
fatigue and malaise, despite antibiotic treatment and intermittent
glucocorticoid therapy. The complete blood count revealed
progressive decrease of the red blood cell count, hemoglobin
concentration and platelet count over 2 weeks. A
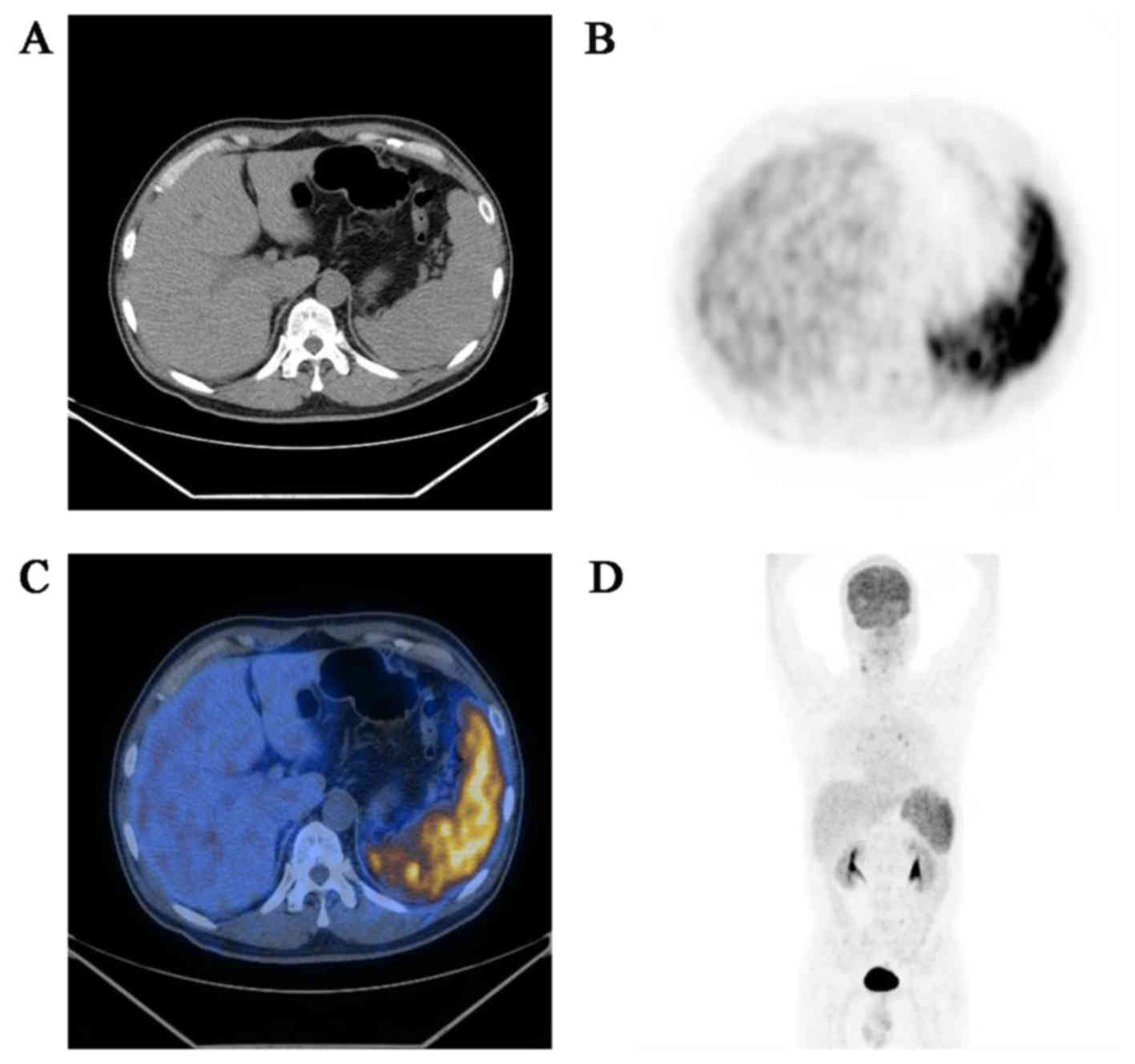
18F-FDG-PET/CT scan was performed to investigate the
possibility of a malignant neoplasm, and it revealed an enlarged
spleen with diffuse increased uptake of 18F-FDG, without
involvement of lymph nodes or other organs (Fig. 1). Diagnostic splenectomy was
performed after obtaining the patient's informed consent. The
histopathological diagnosis was diffuse large B-cell primary
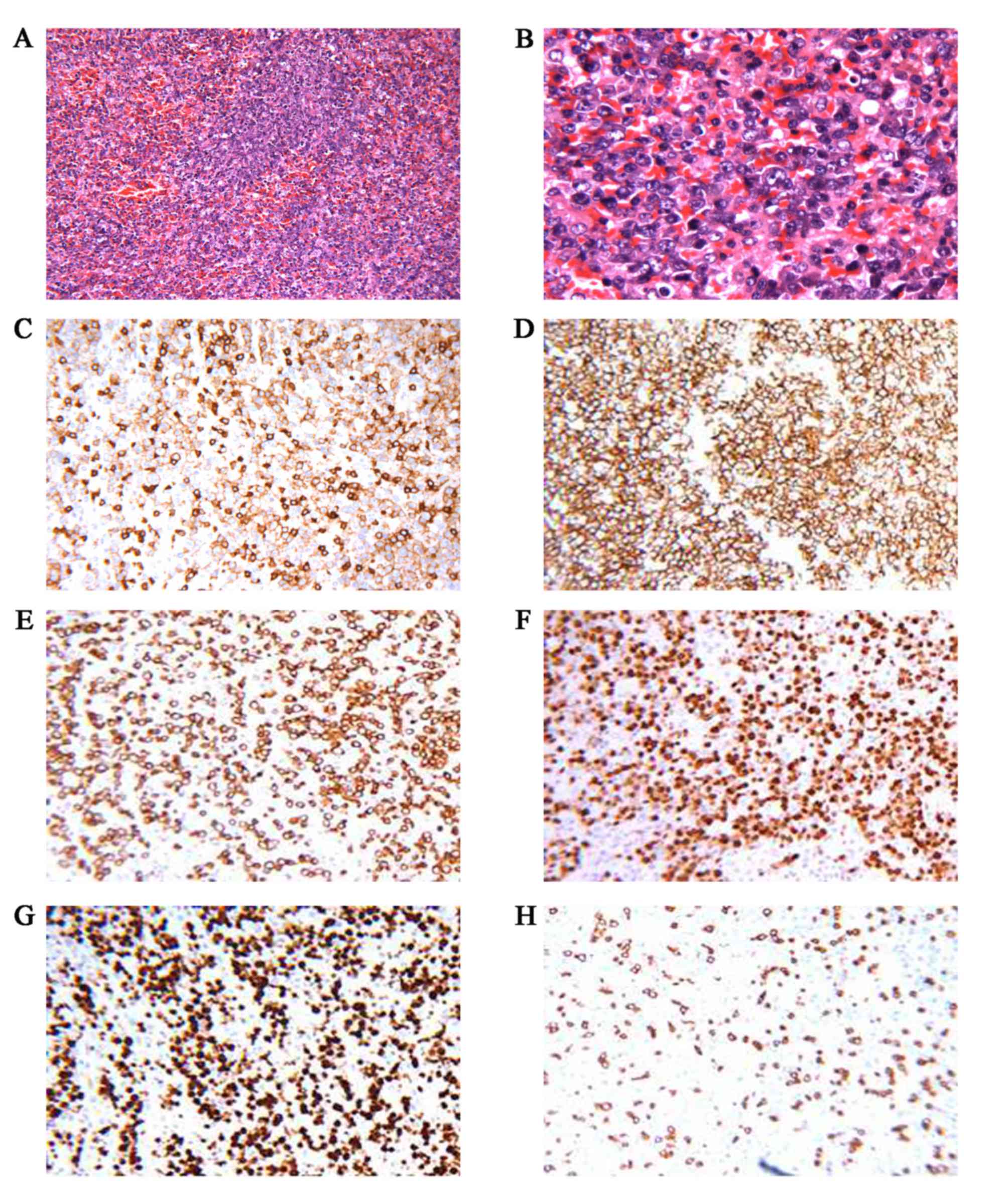
splenic NHL with splenic hilar lymph node involvement (3/3).
Immunohistochemical staining of the tumor cells was positive for
CD20, CD5, multiple myeloma oncogene 1, paired box 5 and B-cell
lymphoma (BCL) 2, and the Ki-67 labeling index was >90%; the
cells were negative for CD3, CD21, CD30, CD10, BCL6 and cyclin D1
(Fig. 2). EBV-encoded RNA was
negative by in situ hybridization of EBV. The patient
received chemotherapy with rituximab, etoposide, cyclophosphamide,
doxorubicin, vincristine and prednisone (R-ECHOP regimen) and
responded well to this treatment. However, after 2 courses of
chemotherapy, the patient developed fever again, with a decrease in
the platelet count, hemoglobin level and granulocyte number, and
abnormal hepatic function. The bone marrow aspiration revealed the
presence of hemophagocytic cells. VP16, dexamethasone and
γ-globulin were administered, as hemophagocytic syndrome was
suspected. The patient's temperature and complete blood count
returned to normal, but the liver function was not restored until
~1 month later, after which time chemotherapy was continued. A
total of 6 chemotherapy cycles were administered and the patient
responded well to this treatment. Until the date of the last
follow-up (May 30, 2016), the patient had not developed any other
discomfort.
Discussion
PSL is a rare malignant disease with an ambiguous
definition, as the spleen is involved in a number of malignancies,
particularly hematological malignancies, such as Hodgkin's lymphoma
and NHL (6,7,10,11). In
addition to the splenic lesions, the strict diagnostic criteria of
PSL include i) splenomegaly, ii) excluding involvement of other
sites by laboratory tests and imaging studies, iii) negative liver
and lymph node biopsies, and iv) disease-free status for ≥6 months
after splenectomy (9). PSL was
grouped into three stages by Ahmann et al (10), depending on the extent of the
disease: In stage I, the tumor is limited to the spleen; in stage
II, the splenic hilum nodes are involved; and stage III includes
involvement of the liver or lymph nodes beyond the splenic hilum;
the patient in our case had stage II disease. The most common
histological subtype of PSLs is large B-cell type (12,13). The
most common presenting symptoms of PSL are fever, malaise, left
upper quadrant pain, weight loss and night sweats (14,15).
Furthermore, during the early stages of the disease, patients tend
to be asymptomatic (16) and
physical examination or laboratory tests usually cannot provide
specific diagnostic information, which greatly increases the
difficulty of diagnosing true PSL. However, imaging studies may
provide more useful information for the diagnosis.
FUO is a common manifestation of a number of
diseases, which are classified into infections, malignancies,
non-infectious inflammatory diseases, miscellaneous causes and
undiagnosed conditions (4,5). Among these etiologies, infection was
the most common cause of FUO in the 1961 survey (1). However, according to Petersdorf's study
published in 1992, neoplastic disease had surpassed infectious
diseases as the etiology of classic FUO (2), while in more recent studies, the
percentage of neoplastic causes has decreased and that of
non-infectious inflammatory diseases and undiagnosed conditions has
increased (5,17–19). The
changes of the etiology proportion of FUO may be attributed to
diagnostic advances, particularly the improvement of imaging and
microbiological studies (4,18,19).
Among the neoplastic causes of FUO, malignant lymphomas are the
most common (20). Besides PSL,
other rare sites of lymphomas manifesting as FUO, such as
intravascular lymphoma (21),
primary central nervous lymphoma (22), colonic lymphoma (23) and pituitary lymphoma (24), have also been reported.
FUO remains a clinical challenge for physicians, as
the overall mortality from FUO is 12–35%, with undiagnosed
conditions accounting for >20% in the 1990s and 2000s, despite
the advances in diagnostic modalities (5).
In the present case, the diagnosis of lymphoma was
suspected prior to splenectomy, as 18F-FDG-PET/CT
revealed an enlarged spleen with increased 18F-FDG
uptake. As reported in the literature, 18F-FDG PET/CT
may help establish the final diagnosis of FUO, as it is sensitive
to the major causes of FUO, including infections, malignancies and
non-infectious inflammatory diseases (25–27). Kim
et al (26) retrospectively
evaluated the role of 18F-FDG PET/CT in the final
diagnosis of FUO and found that it contributed to 65.8% of the
cases. More significantly, for classic FUO patients with elevated
ESR and CRP levels, 18F-FDG PET/CT has a high positive
predictive value (93%) and a high negative predictive value (100%)
(28). In addition,
18F-FDG-PET/CT has also been reported as an important
tool in the diagnosis, staging, detection of recurrence and
monitoring of the treatment response in PSL cases (29–31). A
retrospective study demonstrated that 18F-FDG-PET/CT has
a sensitivity of 96.2%, a specificity of 91.7% and an accuracy of
94% in the diagnosis/staging and restaging of PSL (31). Other studies have reported a
sensitivity of 100% and a specificity of 95–100% of
18F-FDG-PET/CT for the detection of splenic involvement
in malignant lymphoma (32,33).
Splenic fine-needle aspiration (sFNA), splenic core
biopsy (sCB) and splenectomy are the optimal invasive procedure for
diagnosing PSL. Both sFNA and sCB are safe for patients with
splenomegaly, and certain studies reported they exhibit excellent
diagnostic accuracy in splenic malignancies, but a relatively low
efficiency in splenic lymphoma (34,35).
However, other studies indicated they have a rather low diagnostic
yield on account of inadequate specimenin addition, in a review of
49 cases there was an overall complication rate of 16%, and
complications such as bleeding and splenic rupture may have a fatal
outcome (36,37). In patients with diffuse malignant
diseases involving the spleen, splenic puncture may increase the
risk of hemorrhage or rupture (38).
Thus, for patients in a poor condition or those with
contraindications to splenectomy, sFNA and sCB may be feasible
options.
Splenectomy is not only a diagnostic procedure, but
also a therapeutic choice for splenic lymphoma (10,39,40). As
indicated by a retrospective study, splenectomy achieves a high
diagnostic rate in patients with unexplained splenomegaly or
splenic masses (12). In addition,
early splenectomy may reverse cytopenias and significantly improve
the prognosis for PSL or NHL patients, even at an advanced stage
(41,42). Furthermore, splenectomy is considered
to be an effective procedure for establishing the cause of
splenomegaly and FUO, similar to the present case (38).
In addition to splenectomy, treatments for PSL
include systemic chemotherapy and radiotherapy; however,
splenectomy may be preferable, as it provides correct diagnosis and
effective treatment (16).
Splenectomy followed by combination chemotherapy may achieve
excellent long-term survival.
The diagnosis of PSL or the establishment of the
cause of FUO may be difficult. In this rare case of PSL presenting
with FUO and splenomegaly, 18F-FDG-PET/CT and
splenectomy were proven to be of great value in establishing the
final diagnosis. PSL should be taken into consideration in the
differential diagnosis of patients with FUO and splenomegaly.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30900599 and
81470027).
Glossary
Abbreviations
Abbreviations:
|
FUO
|
fever of unknown origin
|
|
PSL
|
primary splenic lymphoma
|
|
FDG
|
fluorodeoxyglucose
|
|
PET
|
positron emission tomography
|
|
CT
|
computed tomography
|
|
NHL
|
non-Hodgkin lymphoma
|
|
H&E
|
hematoxylin and eosin
|
|
sFNA
|
splenic fine-needle aspiration
|
|
sCB
|
splenic core biopsy
|
References
|
1
|
Petersdorf RG and Beeson PB: Fever of
unexplained origin: report on 100 cases. Medicine (Baltimore).
40:1–30. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petersdorf RG: Fever of unknown origin. An
old friend revisited. Arch Intern Med. 152:21–22. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durack DT and Street AC: Fever of unknown
origin-reexamined and redefined. Curr Clin Top Infect Dis.
11:35–51. 1991.PubMed/NCBI
|
|
4
|
Hayakawa K, Ramasamy B and Chandrasekar
PH: Fever of unknown origin: an evidence-based review. Am J Med
Sci. 344:307–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mourad O, Palda V and Detsky AS: A
comprehensive evidence-based approach to fever of unknown origin.
Arch Intern Med. 163:545–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brox A and Shustik C: Non-Hodgkin's
lymphoma of the spleen. Leuk Lymphoma. 11:165–171. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gobbi PG, Grignani GE, Pozzetti U,
Bertoloni D, Pieresca C, Montagna G and Ascari E: Primary splenic
lymphoma: Does it exist? Haematologica. 79:286–293. 1994.PubMed/NCBI
|
|
8
|
Rueffer U, Sieber M, Stemberg M, Gossmann
A, Josting A, Koch T, Grotenhermen F and Diehl V: German Hodgkin's
Lymphoma Study Group (GHSG): Spleen involvement in Hodgkin's
lymphoma: assessment and risk profile. Ann Hematol. 82:390–396.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasgupta T, Coombes B and Brasfield RD:
Primary malignant neoplasms of the spleen. Surg Gynecol Obstet.
120:947–960. 1965.PubMed/NCBI
|
|
10
|
Ahmann DL, Kiely JM, Harrison EG Jr and
Payne WS: Malignant lymphoma of the spleen. A review of 49 cases in
which the diagnosis was made at splenectomy. Cancer. 19:461–469.
1966. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Straus DJ, Filippa DA, Lieberman PH,
Koziner B, Thaler HT and Clarkson BD: The non-Hodgkin's lymphomas.
I. A retrospective clinical and pathologic analysis of 499 cases
diagnosed between 1958 and 1969. Cancer. 51:101–109. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kraus MD, Fleming MD and Vonderheide RH:
The spleen as a diagnostic specimen: a review of 10 years'
experience at two tertiary care institutions. Cancer. 91:2001–2009.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizu-Kohno K, Kimura Y, Kiyasu J,
Miyoshi H, Yoshida M, Ichikawa R, Niino D and Ohshima K: Malignant
lymphoma of the spleen in Japan: a clinicopathological analysis of
115 cases. Pathol Int. 62:577–582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spier CM, Kjeldsberg CR, Eyre HJ and Behm
FG: Malignant lymphoma with primary presentation in the spleen. A
study of 20 patients. Arch Pathol Lab Med. 109:1076–1080.
1985.PubMed/NCBI
|
|
15
|
Harris NL, Aisenberg AC, Meyer JE, Ellman
L and Elman A: Diffuse large cell (histiocytic) lymphoma of the
spleen. Clinical and pathologic characteristics of ten cases.
Cancer. 54:2460–2467. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han SM, Teng CL, Hwang GY, Chou G and Tsai
CA: Primary splenic lymphoma associated with hemophagocytic
lymphohistiocytosis complicated with splenic rupture. J Chin Med
Assoc. 71:210–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Efstathiou SP, Pefanis AV, Tsiakou AG,
Skeva II, Tsioulos DI, Achimastos AD and Mountokalakis TD: Fever of
unknown origin: discrimination between infectious and
non-infectious causes. Eur J Intern Med. 21:137–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaya A, Ergul N, Kaya SY, Kilic F, Yilmaz
MH, Besirli K and Ozaras R: The management and the diagnosis of
fever of unknown origin. Expert Rev Anti Infect Ther. 11:805–815.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mete B, Vanli E, Yemisen M, Balkan II,
Dagtekin H, Ozaras R, Saltoglu N, Mert A, Ozturk R and Tabak F: The
role of invasive and non-invasive procedures in diagnosing fever of
unknown origin. Int J Med Sci. 9:682–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roca Campañá V and Rodríguez Silva H:
Malignant lymphomas presenting as fever of unknown origin. An Med
Interna. 24:531–534. 2007.(In Spanish). PubMed/NCBI
|
|
21
|
Zeidman A, Horowitz A, Fradin Z, Cohen A,
Wolfson L and Elimelech O: Fulminant intravascular lymphoma
presenting as fever of unknown origin. Leuk Lymphoma. 45:1691–1693.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salih SB, Saeed AB, Alzahrani M, Al QM,
Haider A and Palker V: Primary CNS lymphoma presenting as fever of
unknown origin. J Neurooncol. 93:401–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blanco S Casallo, de Matías Salces L,
Sánchez F Marcos, Martín Barranco MJ, Núñez Cuerda E and Solano
Ramos F: Colon lymphoma as a cause of fever of unknown origin. An
Med Interna. 23:379–381. 2006.PubMed/NCBI
|
|
24
|
Landman RE, Wardlaw SL, McConnell RJ,
Khandji AG, Bruce JN and Freda PU: Pituitary lymphoma presenting as
fever of unknown origin. J Clin Endocrinol Metab. 86:1470–1476.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meller J, Sahlmann CO and Scheel AK:
18F-FDG PET and PET/CT in fever of unknown origin. J
Nucl Med. 48:35–45. 2007.PubMed/NCBI
|
|
26
|
Kim YJ, Kim SI, Hong KW and Kang MW:
Diagnostic value of 18F-FDG PET/CT in patients with
fever of unknown origin. Intern Med J. 42:834–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu L and Chen Y: The role of
18F-FDG PET or PET/CT in the detection of fever of
unknown origin. Eur J Radiol. 81:3524–3529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balink H, Collins J, Bruyn GA and Gemmel
F: F-18 FDG PET/CT in the diagnosis of fever of unknown origin.
Clin Nucl Med. 34:862–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karunanithi S, Roy SG, Murugan V, Bal C
and Kumar R: 18F-FDG-PET/CT in staging, recurrence
detection and response evaluation of primary splenic lymphoma with
eight years follow up. Nucl Med Rev Cent East Eur. 18:37–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takata F, Kaida H, Ishibashi M, Kurata S,
Uozumi J, Uchida M, Okamura T, Uchida S, Oshima K, Ban S, et al:
Primary splenic lymphoma detected by F-18 FDG PET. Clin Nucl Med.
33:204–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karunanithi S, Sharma P, Roy SG, Vettiyil
B, Sharma A, Thulkar S, Bal C and Kumar R: Use of
18F-FDG PET/CT imaging for evaluation of patients with
primary splenic lymphoma. Clin Nucl Med. 39:772–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rini JN, Leonidas JC, Tomas MB and
Palestro CJ: 18F-FDG PET versus CT for evaluating the
spleen during initial staging of lymphoma. J Nucl Med.
44:1072–1074. 2003.PubMed/NCBI
|
|
33
|
de Jong PA, van Ufford HM, Baarslag HJ, de
Haas MJ, Wittebol SH, Quekel LG and de Klerk JM: CT and
18F-FDG PET for noninvasive detection of splenic
involvement in patients with malignant lymphoma. AJR Am J
Roentgenol. 192:745–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tam A, Krishnamurthy S, Pillsbury EP,
Ensor JE, Gupta S, Murthy R, Ahrar K, Wallace MJ, Hicks ME and
Madoff DC: Percutaneous image-guided splenic biopsy in the oncology
patient: an audit of 156 consecutive cases. J Vasc Interv Radiol.
19:80–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Civardi G, Vallisa D, Berté R, Giorgio A,
Filice C, Caremani M, Caturelli E, Pompili M, De Sio I, Buscarini E
and Cavanna L: Ultrasound-guided fine needle biopsy of the spleen:
high clinical efficacy and low risk in a multicenter Italian study.
Am J Hematol. 67:93–99. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lishner M, Lang R, Hamlet Y, Halph E,
Steiner Z, Radnay J and Ravid M: Fine needle aspiration biopsy in
patients with diffusely enlarged spleens. Acta Cytol. 40:196–198.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lal A, Ariga R, Gattuso P, Nemcek AA and
Nayar R: Splenic fine needle aspiration and core biopsy. A review
of 49 cases. Acta Cytol. 47:951–959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han B, Yang Z, Yang T, Gao W, Sang X, Zhao
Y and Shen T: Diagnostic splenectomy in patients with fever of
unknown origin and splenomegaly. Acta Haematol. 119:83–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grosskreutz C, Troy K and Cuttner J:
Primary splenic lymphoma: Report of 10 cases using the Real
classification. Cancer Invest. 20:749–753. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kehoe J and Straus DJ: Primary lymphoma of
the spleen. Clinical features and outcome after splenectomy.
Cancer. 62:1433–1438. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morel P, Dupriez B, Gosselin B, Fenaux P,
Estienne MH, Facon T, Jouet JP and Bauters F: Role of early
splenectomy in malignant lymphomas with prominent splenic
involvement (primary lymphomas of the spleen). A study of 59 cases.
Cancer. 71:207–215. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lehne G, Hannisdal E, Langholm R and Nome
O: A 10-year experience with splenectomy in patients with malignant
non-Hodgkin's lymphoma at the norwegian radium hospital. Cancer.
74:933–939. 1994. View Article : Google Scholar : PubMed/NCBI
|