Introduction
The heterogeneous course of adenocarcinoma of the
colon has prompted the search for new prognostic and diagnostic
tools. As a result of this search, angiogenesis in the primary
tumor was revealed to be necessary for tumor progression. In
addition, the heterogeneity in large intestinal adenocarcinomas has
been attributed to the level of angiogenesis. Therefore, an
improved understanding of the process of angiogenesis may provide
novel anticancer therapies, as well as new prognostic and
predictive tools.
In tumors, an autonomous system of blood vessels
develops under the strict control of stimulating and inhibiting
factors (1,2). A key player at all stages of
angiogenesis is the signaling molecule, vascular endothelial growth
factor (VEGF). VEGF belongs to the family of platelet-derived
growth factors, which includes VEGF-A, VEGF-B, VEGF-C, VEGF-D,
VEGF-E and placental growth factor (3,4).
However, the predominant factor promoting the formation and growth
of new vessels is VEGF-A (5). VEGF
exerts its biological effects through the following glycoprotein
tyrosine kinase receptors: VEGFR-1 (also known as the Fms-like
tyrosine kinase, Flt-1); VEGFR-2 (also termed fetal liver kinase,
Flk-1) and VEGFR-3 (6,7). In particular, Flt-1 and Flk-1 are the
two receptors directly involved in the formation of blood vessels
(8). Although the role of Flk-1 is
well understood (i.e., it transduces the stimulating signal into
the cell through the activation of the tyrosine kinase cascade),
the role of Flt-1 in the angiogenic process has yet to be properly
elucidated.
Flt-1 is involved in both inflammation and
carcinogenesis. Expression of Flt-1 is not restricted to vascular
endothelial cells. It is also found on cells of hematopoietic
lineage (i.e., monocytes and macrophages), where it has a
regulatory function. For example, Flt-1 has been shown to be
involved in the mobilization of macrophages, and it is able to
induce macrophage cytokine secretion (9). Additionally, Flt-1 is expressed on
dendritic cells, osteoclasts, pericytes, hepatocytes, trophoblast
cells of the placenta (10) and
smooth muscle cells (11). With
respect to carcinogenesis, activation of Flt-1 may affect tumor
development multidirectionally. It contributes to the proliferation
and migration of vascular endothelial cells and tumor cells.
Furthermore, Flt-1 has been revealed to support the phenotypic
change of cancer cells into mobile units that are capable of
migration, a process called epithelial-mesenchymal transition (EMT)
(12). Flt-1 is also involved in the
preparation of the pre-metastatic niche, i.e., a
‘metastasis-friendly’ environment. In the first stage of the niche
formation, fibroblasts produce and secrete fibronectin, which is a
target for migration of the hematopoietic progenitor cells
(monocytes and macrophages) released from the bone marrow that
contain Flt-1. These monocytes and macrophages are subsequently
able to mobilize tumor cells, thereby creating a
‘metastasis-friendly’ environment (13,14).
The present study aimed to determine whether
pro-angiogenic factors (i.e., VEGF and Flt-1), as well as
angiogenesis itself [measured by the microvessel density (MVD)] in
the tumor, contribute to the pathology and prognosis of patients
with resectable colorectal cancer.
Materials and methods
Patients and tissue samples
The present study was a retrospective study of 139
patients who underwent surgery in the Clinic of Oncologic Surgery,
Medical University of Gdansk, Poland, between September 1998 and
December 2002. For the immunohistochemistry staining, archived
tissue material from primary tumors obtained during surgery was
used. The pathoclinical characteristics of the study group are
presented in Table I. Patients were
not subjected to oncological treatment prior to surgery. The
operation met the criteria of R0 resection, i.e., it was locally
oncologically radical. The stage of cancer was determined according
to the pathological tumor-lymph nodes-metastasis (pTNM)
classification system (15).
Histological evaluation was based on the World Health Organization
classification (16). In all cases,
adenocarcinoma was diagnosed, and well (G1), moderately (G2) and
poorly (G3) differentiated tumors were differentiated. The minimum
follow-up of patients remaining alive was 44 months. The study was
approved by the Independent Bioethical Committee for Scientific
Research, no. NKEBN/4/005.
 | Table I.Characteristics of patients in the
study group (n=139). |
Table I.
Characteristics of patients in the
study group (n=139).
| Parameter | No. of patients
(%) |
|---|
| Clinical stage
according to: |
|
| pTNM |
|
| I | 15 (10.8) |
| II | 47 (33.8) |
| III | 48 (34.5) |
| IV | 29 (20.9) |
| pT feature |
|
| 1 | 2 (1.58) |
| 2 | 21 (15.1) |
| 3 | 96 (69.0) |
| 4 | 20 (14.4) |
| pN feature |
|
| 0 | 68 (48.9) |
| 1 | 43 (30.9) |
| 2 | 28 (20.2) |
| pM feature |
|
| 0 | 110 (79.1) |
| 1 | 29 (20.9) |
| Grade |
|
| G1 | 21 (15.1) |
| G2 | 106 (76.3) |
| G3 | 12 (8.6) |
| Location |
|
|
Rectum | 61 (43.9) |
|
Colon | 78 (56.1) |
| Gender |
|
|
Female | 61 (43.9) |
| Male | 78 (56.1) |
| Age (median, 66
years) |
|
|
≥Median | 78 (56) |
|
<Median | 61 (44) |
Immunohistochemistry
The tissues were fixed in 4% formaldehyde solution,
dehydrated with ethyl alcohol, and embedded in low-melting
paraffin. Paraffin blocks were subsequently processed and cut on
the sledge microtome into 4-µm sections. The sections were
routinely stained with hematoxylin and eosin. Tumor fragments
without necrosis were selected for the present study.
Representative sections were applied on to glass slides coated with
2% silane solution (APES; cat. no. A3648, Sigma-Aldrich, St. Louis,
MO, USA). Sections were incubated at 36°C for 24 h, deparaffinized
and rehydrated. The following antibodies were used: Polyclonal
anti-Flt-1 (c-17) rabbit antibody (cat. no. sc-316; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), polyclonal anti-VEGF (A-20)
rabbit antibody (cat. no. Sc-152; Santa Cruz Biotechnology), and
monoclonal anti-CD34 mouse antibody (cat. no. M7165; Dako,
Carpinteria, CA, USA). Determination of antigen expression was
performed according to the antibodies' manufacturers'
protocols.
Vascular density determination using
anti-CD34 antibodies
The slides were subjected to heat treatment in a
water bath in Target Retrieval Solution (cat. no. S1700; pH 6.0,
Dako) at a temperature of 99°C for 20 min. Subsequently, slides
were cooled at room temperature for 20 min and washed in
phosphate-buffered saline (PBS) twice for 10 min. They were
subsequently immersed in a 3% solution of hydrogen peroxide for a
further 10 min, prior to being washed twice for 10 min in PBS. The
primary anti-CD34 antibodies (cat. no. M 7165; Dako) were applied
to the slides at a dilution of 1:25, and incubated for 1 h at room
temperature. Following incubation, the slides were washed twice in
PBS for 10 min. Biotinylated anti-mouse or anti-rabbit linking
antibodies (Dako; cat. no. K675) were subsequently applied to the
slides, and incubated at room temperature for 30 min. Following
incubation, the slides were washed twice in PBS for 10 min prior to
streptavidin-conjugated horseradish peroxidase (Dako; cat. no.
K675) being applied and incubated at room temperature for 30 min.
The slides were washed twice in PBS for 10 min, subsequently
immersed in a substrate solution of diaminobenzidine (cat. no.
K3468; Dako), and further incubated at room temperature for 10 min.
The slides were washed with running water for 10 min, stained with
Mayer's hematoxylin (Sigma-Aldrich) for 5 min, and subsequently
washed again in running tap-water for 10 min. The procedure ended
with dehydration of the preparation, clearing and mounting it with
Canada balsam (Avantor Performance Materials Poland S.A., Gliwice,
Poland). Finally, in order to assess the MVD in the slides stained
for CD34, areas of increased vascularity (‘hot spots’) were
searched at ×40 and ×100 magnification, according to the procedure
described by Weidner et al (17). The MVD calculation was performed at
×200 magnification in the area of 0.785 mm2.
VEGF expression determination using an
anti-VEGF antibody
The slides were prepared as described for the anti
CD-34 antibodies, except that the primary anti-VEGF antibodies
(A-20; cat. no. sc-152; Santa Cruz Biotechnology) were applied at a
dilution of 1:100 and incubated for 2 h at room temperature. VEGF
expression in tumor cells was evaluated on a two-point scale of the
reaction intensity, depending on the resulting color reaction
(i.e., 0 for no reaction or a weak reaction, indicating no
expression of VEGF, and 1 for an intense reaction, indicating
overexpression of VEGF).
Flt-1 expression determination using
an anti-Flt-l antibody
The slides were prepared as described for the anti
CD-34 antibodies, except that the primary Flt-l antibody (cat. no.
c-l7; Santa Cruz Biotechnology) was applied at a dilution of 1:300
and incubated for 2 h at room temperature. Flt-1 expression in
tumor cells was evaluated on a two-point scale of the reaction
intensity, depending on the resulting color reaction (i.e., 0 for
no reaction, indicating no Flt-1 expression, and 1 for a reaction,
indicating Flt-1 expression).
Statistical analysis
The association among VEGF and Flt-1 expression, MVD
and overall survival in months, median age, gender, location of the
tumor (colon vs. rectum), the extent of tumor infiltration (pT),
status of regional lymph nodes (pN), the presence of distant
metastases (pM), pTNM staging, and tumor grade (G1-G3) were
assessed. Statistical analysis was performed using the data
analysis software system, STATISTICA tools, version 10 (www.statsoft.com; StatSoft, Inc., 2011). To determine
the correlation between MVD in tumor stroma and the expression of
VEGF and Flt-1, or the association between the MVD and
pathoclinical tumor parameters, the Mann-Whitney U and the
Kruskal-Wallis analysis of variance (ANOVA) tests were used.
Assessment of the correlation between VEGF and Flt-1 expression and
tumor pathoclinical parameters was performed using Pearson's
χ2 test. Survival analysis was performed using the
Kaplan-Meier method. Differences between survival times in the
studied groups were verified using the log-rank test. For all
calculations, P<0.05 was considered to indicate a statistically
significant value. In certain cases, for the purpose of statistical
analysis, the groups of evaluated pathoclinical parameters were
combined due to their small size (i.e., T1+T2 vs. T3+T4, and stages
I+II vs. stages III+IV).
Results
Correlation between MVD and VEGF or
Flt-1 expression
The average MVD in the patient tissue samples was
30.3 microvessels (median, 27.5 microvessels) in the field of view.
MVD ranged from 5–80 microvessels/field of view (standard
deviation, 15 microvessels/field of view). Overexpression of VEGF
was identified in 73 (52.5%) cases of colorectal cancer in the
present study. In the remaining 66 tumors that were analyzed
(47.5%), no VEGF expression was detected; nor it was expressed at
very low levels. Flt-1 expression was detected in 102 (73%) primary
colorectal tumors, whereas 37 (27%) tumors revealed no Flt-1
expression. Subsequently, the correlation between the MVD in the
primary tumor and the expression level of VEGF or Flt-1 in the
tumor cells was analyzed (Table
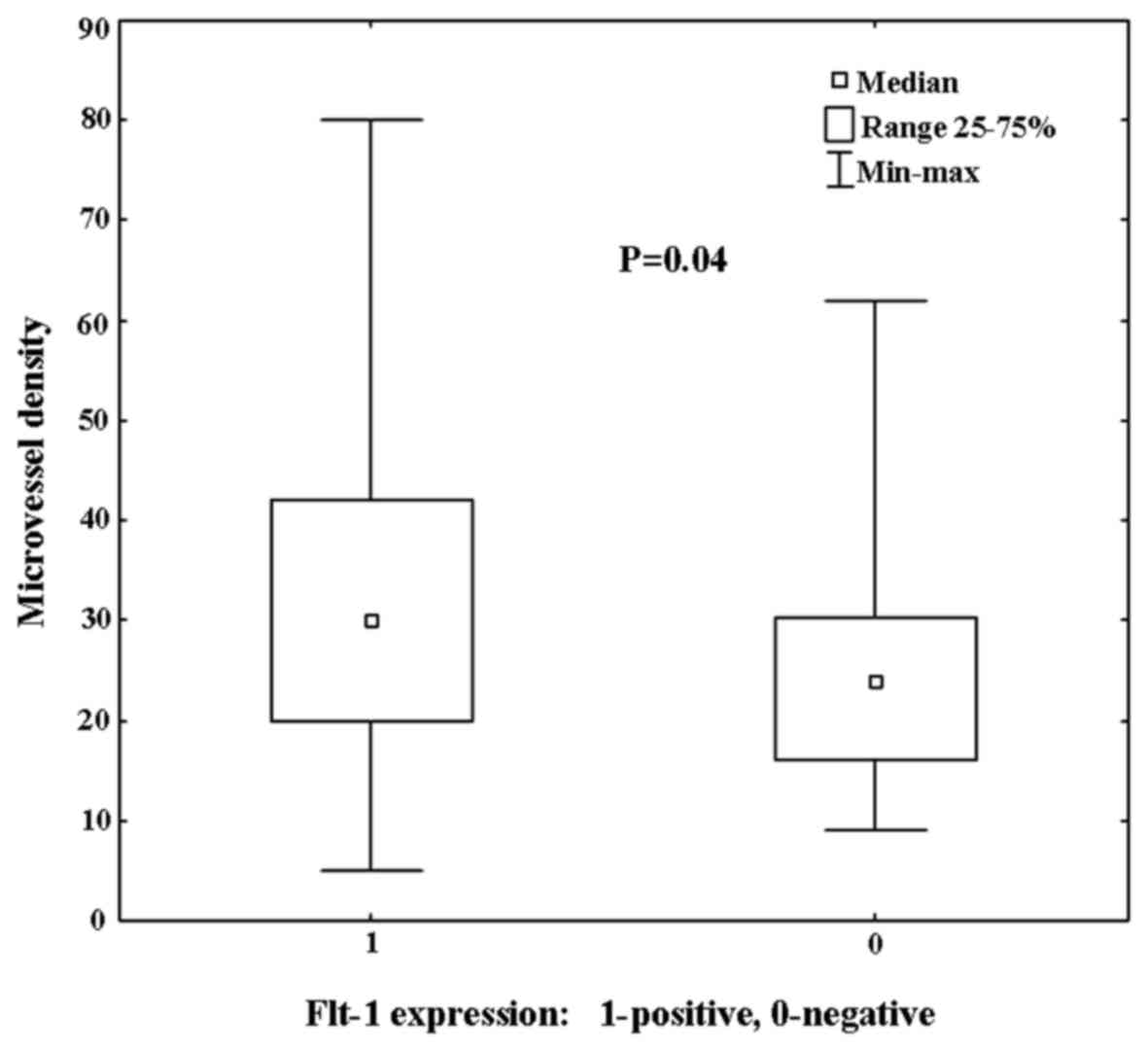
II). Significantly higher vascular density in tumors with
positive expression of the Flt-1 was observed (P=0.04; Fig. 1). Furthermore, a higher MVD in the
tumor stroma in tumors with VEGF overexpression was also observed;
however, this correlation was not statistically significant
(P=0.6).
 | Table II.The relationship between VEGF or Flt1
expression and the microvessel density in the study group
(n=139). |
Table II.
The relationship between VEGF or Flt1
expression and the microvessel density in the study group
(n=139).
|
Protein/expression | Median microvessel
density in the tumor (microvessels/field of view) |
P-valuea |
|---|
| Flt1 |
| 0.04 |
|
Positive expression | 30 |
|
| No
expression | 24 |
|
| VEGF |
| ns |
|
Overexpression | 30 |
|
| No
expression | 25.5 |
|
Correlation between colorectal cancer
pathoclinical parameters and MVD, VEGF or Flt-1 expression
No significant correlation was identified between
MVD and the pathoclinical parameters of colorectal cancer in the
present study (P>0.05, according to the Mann-Whitney U and
Kruskal-Wallis ANOVA tests). Furthermore, no statistically
significant correlation was identified among the pathoclinical
parameters of colorectal cancer, including age, gender, location of
the tumor, stage or grade, and the intensity of VEGF expression in
cancer cells. Similarly, statistically significant correlation was
identified between these parameters and Flt-1 expression in the
analyzed tumors (P>0.05, according to Pearson's χ2
test). However, a significant correlation between VEGF
overexpression in tumor cells and Flt-1 expression was identified.
In tumors with Flt-1 expression (n=102), 59 (58%) also revealed
overexpression of VEGF, whereas 43 (42%) Flt-1 positive tumors had
no VEGF expression (P=0.03, according to Pearson's χ2
test).
Correlation between overall survival
and MVD, VEGF or Flt-1 expression
A survival analysis was performed on the 139
patients included in the present study on the basis of VEGF or
Flt-1 expression, MVD, and selected pathoclinical parameters of
colorectal cancer (Table III). The
intensity of VEGF and Flt-1 expression, and of MVD in the tumor
stroma did not correlate with the prognosis in patients with
colorectal cancer, as measured by overall survival. Patients with
colon cancer had a significantly better prognosis (P=0.03). In
addition, patients with metastases in the regional lymph nodes (pN)
and distant metastases (pM) had a significantly poorer prognosis
(P=0.03 and P<0.01, respectively).
 | Table III.Effect of vascular growth factors,
microvessel density, and selected pathoclinical parameters of
colorectal cancer on overall survival time in the study group
(n=139). |
Table III.
Effect of vascular growth factors,
microvessel density, and selected pathoclinical parameters of
colorectal cancer on overall survival time in the study group
(n=139).
| Parameter |
P-valuea |
|---|
| Gender | ns |
|
Female |
|
|
Male |
|
| Age (median, 66
years) | ns |
|
<Median |
|
|
≥Median |
|
| Location | 0.03 |
|
Colon |
|
|
Rectum |
|
| pT feature | ns |
| T1 +
T2 |
|
| T3 +
T4 |
|
| pN feature | 0.03 |
| N0 |
|
| N+ |
|
| pM feature | <0.01 |
| M0 |
|
| M+ |
|
| Stage | 0.01 |
| I +
II |
|
| III +
IV |
|
| Grade | ns |
| G1 |
|
| G2 |
|
| G3 |
|
| Flt1 | ns |
|
Positive expression |
|
| No
expression |
|
| VEGF | ns |
|
Overexpression |
|
| No
expression |
|
| Microvessels/field
of view | ns |
|
<Median |
|
|
≥Median |
|
Discussion
Anti-angiogenic therapy has proven beneficial in the
treatment of advanced colorectal cancer (18). However, in the present study, no
significant correlations were identified between the pathoclinical
parameters of colorectal cancer and stromal vascular density.
Furthermore, no differences were identified between the vascular
density in patients differing in their T, N and M classification,
or in their tumor grade. Similarly, no differences were identified
in vascular density in the primary tumor with respect to
characteristics including age, gender, and tumor location.
Although our results showing no correlation between
MVD and colorectal cancer parameters are similar to findings
previously reported by British and Japanese researchers (19,20), to
date, it has not been conclusively determined whether the MVD in
the primary tumors of colorectal cancer affects the prognosis of
the disease. Indeed, in esophageal cancer, Kitadai et al
(21) reported a correlation between
increased MVD in the primary tumor, which was assessed with
anti-CD34, and poor prognosis and early local recurrence (21). Similarly, in gastric cancer, high
vascular density correlated with a poorer prognosis in early
(22) and in advanced (23) cancers of the stomach. However, in the
case of colorectal cancer, no such conclusive assessments were
drawn. Similarly to the present study, other reports have indicated
that no correlation exists between MVD in the primary tumor, as
assessed with anti-CD34, and patient prognosis (24,25). On
the other hand, certain studies have demonstrated that the MVD in
the primary tumor of colorectal cancer, which was also assessed
using anti-CD34, correlates with a poorer prognosis (26,27).
Previous reports have also demonstrated a positive effect of an
increased MVD, which was determined using anti-CD31 and antibodies
against the von Willebrand factor in the primary tumor of
colorectal cancer on prognosis (28,29).
These discrepancies in the effects of MVD on
colorectal cancer prognosis may be attributed to the various
markers that were used to identify the vascular endothelium. In the
present study, the anti-CD34 antibody was used due to its high
sensitivity and the reproducibility of the obtained results
(30). However, anti-CD34 reacts
with both active and inactive vascular endothelial cells, and
therefore it is not a marker of endothelial cell proliferation.
However, it may be used to detect endothelial cells that are
‘trapped’ in the tumor cells. Another commonly used antibody for
determining the vascular density is an antibody raised against the
von Willebrand factor (31).
However, this antigen is not present in all endothelial cells, and
it is also present on platelets. Finally, an appreciable number of
studies have used anti-CD31 or anti-CD105 glycoprotein antibodies.
The first antibody identifies endothelial cells, although it is
also present on certain lineages of leukocytes, whereas the latter
only reacts with activated endothelial cells and is therefore a
marker associated with proliferation (32,33).
Thus, the MVD measurements using these above-mentioned markers are
subject to considerable risk of error, depending on the antibodies
used. Additionally, the risk increases with the subjectivity of the
method for evaluating the MVD (30).
The expression of two growth factors, VEGF and
Flt-1, was studied, and, as anticipated, a positive correlation
between Flt-1 expression and increased MVD in the tumor was
demonstrated. There was also a clear trend for a similar
association between VEGF expression and increased MVD. However, no
correlation was identified between VEGF expression and the
potential prognostic pathoclinical parameters describing patients
with colorectal cancer. By contrast, the majority of reports
concerning colorectal cancer have indicated that the overexpression
of VEGF is associated with poor patient prognosis (34). Furthermore, this association between
VEGF expression and poor prognosis was consistent for groups where
only colon cancer was evaluated, as well as for those where
patients with rectal and colon cancer were evaluated together. On
the other hand, several reports have corroborated the observations
presented in the current study that there is no link between VEGF
overexpression and prognosis in colorectal cancer (34).
When interpreting the results in the present study,
it should be kept in mind that VEGF was only assessed in the
primary tumor. As the primary tumor is undergoing dynamic growth,
there may be multiple modes of angiogenesis occurring. Indeed,
tumors frequently use more than one strategy to acquire vessels,
depending on the tumor stage and grade. Additionally, VEGF
expression is not an easy parameter to determine. Generally,
immunohistochemical methods using antibodies are used to identify
VEGF, and its presence is measured by the intensity of a color
reaction, which may also occur, for example, in damaged cells
(35). However, there is no uniform,
standardized method for assessing the level of VEGF expression.
Indeed, the intensity of the color reaction as a measure of VEGF
expression may be presented on different scales. In the present
study, the analyses were simplified to a two-point scale, i.e., the
lack of a reaction, or a weak positive reaction, indicated the lack
of VEGF expression, whereas a strong positive reaction indicated
that VEGF was overexpressed. Indeed, the prognostic value of VEGF
assessment is a topic of debate due to the ambiguous, and often
contradictory, research findings (36).
Tumor heterogeneity and inadequacies of the
immunohistochemical methods employed may also have affected the
assessment of Flt-1 expression and its correlation with the
pathoclinical parameters of colorectal cancer reported in the
present study. Although EMT occurs in the tumor itself and is
involved with tumor cells, the formation of a pre-metastatic niche
involving Flt-1 occurs in the place where, subsequently, metastasis
from the original site will develop, and is formed by precursor
cells migrating from the bone marrow. Therefore, the expression of
Flt-1 should also be determined in cells of the liver, lungs and in
other regions, as well as the primary tumor (37). Inflammatory cells expressing Flt-1
are similarly dispersed, which are conducive to immune tolerance to
cancer. Thus, the large dispersion of cells expressing Flt-1 in the
body, and the diverse involvement of Flt-1 in the process of
tumorigenesis, indicate that its expression in the tumor alone does
not reflect the actual role of Flt-1. In a group of 58 patients
with colon cancer and 10 patients with colorectal adenoma, no
differences in Flt-1 expression were identified between adenoma and
carcinoma patients, or, as reported in the present study, between
the particular tumor stages (38).
It was hypothesized that Flt-1 expression in the primary tumor
should favor metastasis, therefore leading to a poorer prognosis.
However, in our study group, no such correlations were identified.
Indeed, no correlation between Flt-1 expression and the markers of
poor prognosis in patients (i.e., the presence of metastasis, or a
shorter survival following surgery) were observed. On the other
hand, in several publications associated with colorectal cancer,
marked Flt-1 expression was a marker of poor prognosis in patients.
In a study on 91 patients with colon and rectal cancer, high Flt-1
expression correlated with the shorter post-operative survival of
patients with clinical stage II and III cancer (39). In another study of 140 patients with
colon cancer, Flt-1 overexpression was predictive of early local
recurrence (40). It is worth
noting, however, that immunohistochemical methods are not perfect,
and, as mentioned above, the diverse role of Flt-1 and its
dispersion throughout the body may have led to an inadequate
assessment of its pathoclinical role in colorectal cancer. Such
inadequacies may explain the significant differences in the results
achieved by various investigators.
In conclusion, in the present study an increased
expression of VEGF and Flt-1 receptor was shown to be associated
with increased MVD in the primary tumor in resectable colorectal
cancer. However, neither the vascular density in the primary tumor,
nor the expression of VEGF and Flt-1, correlated with potentially
prognostic pathoclinical factors and overall survival in resectable
colorectal cancer.
Acknowledgements
Language assistance in the preparation of this
manuscript was kindly provided by Proper Medical Writing Sp. z
o.o., Warsaw, Poland.
References
|
1
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinkaruk S, Bayle M, Laïn G and Déléris
G: Vascular endothelial cell growth factor (VEGF), an emerging
target for cancer chemotherapy. Curr Med Chem Anticancer Agents.
3:95–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor: Basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofstaetter JG, Saad FA, Samuel RE,
Wunderlich L, Choi YH and Glimcher MJ: Differential expression of
VEGF isoforms and receptors in knee joint menisci under systemic
hypoxia. Biochem Biophys Res Commun. 324:667–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibuya M: Structure and function of
VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct
Funct. 26:25–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rmali K, Puntis M and Jiang W:
Tumour-associated angiogenesis in human colorectal cancer.
Colorectal Dis. 9:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz JD, Rowinsky EK, Youssoufian H,
Pytowski B and Wu Y: Vascular endothelial growth factor receptor-1
in human cancer: Concise review and rationale for development of
IMC-18F1 (Human antibody targeting vascular endothelial growth
factor receptor-1). Cancer. 116:(Suppl 4). S1027–S1032. 2010.
View Article : Google Scholar
|
|
10
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen
P, Rafii S and Hicklin DJ: The vascular endothelial growth factor
receptor (VEGFR-1) supports growth and survival of human breast
carcinoma. Int J Cancer. 119:1519–1529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lesslie DP, Summy JM, Parikh NU, Fan F,
Trevino JG, Sawyer TK, Metcalf CA, Shakespeare WC, Hicklin DJ,
Ellis LM and Gallick GE: Vascular endothelial growth factor
receptor-1 mediates migration of human colorectal carcinoma cells
by activation of Src family kinases. Br J Cancer. 94:1710–1717.
2006.PubMed/NCBI
|
|
13
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaplan RN, Rafii S and Lyden D: Preparing
the ‘soil’: The premetastatic niche. Cancer Res. 66:11089–11093.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Handbook. 7th.
Springer; New York: 2010
|
|
16
|
Hamilton SR and Aaltonen LA: Pathology and
genetics of tumours of the digestive system. IARC Press; Lyon:
2000
|
|
17
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Eng J Med. 324:1–8. 1991. View Article : Google Scholar
|
|
18
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III:
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duff SE, Jeziorska M, Kumar S, Haboubi N,
Sherlocks D, O'Dwyer ST and Jayson GC: Lymphatic vessel density,
microvessel density and lymphangiogenic growth factor expression in
colorectal cancer. Colorectal Dis. 9:793–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teranishi N, Naito Z, Ishiwata T, Tanaka
N, Furukawa K, Seya T, Shinji S and Tajiri T: Identification of
neovasculature using nestin in colorectal cancer. Int J Oncol.
30:593–603. 2007.PubMed/NCBI
|
|
21
|
Kitadai Y, Haruma K, Tokutomi T, Tanaka S,
Sumii K, Carvalho M, Kuwabara M, Yoshida K, Hirai T, Kajiyama G and
Tahara E: Significance of vessel count and vascular endothelial
growth factor in human esophageal carcinomas. Clin Cancer Res.
4:2195–2200. 1998.PubMed/NCBI
|
|
22
|
Xiangming C, Hokita S, Natsugoe S, Tanabe
G, Baba M, Takao S, Kuroshima K and Aikou T: Angiogenesis as an
unfavorable factor related to lymph node metastasis in early
gastric cancer. Ann Surg Oncol. 5:585–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isozaki H, Okajima K, Kawashima Y, Yamada
S, Nakata E, Nishimura J and Ichinona T: Prognostic value of the
number of metastatic lymph nodes in gastric cancer with radical
surgery. J Surg Oncol. 53:247–251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gallego M Galindo, Fernández Acenero MJ,
Ortega J Sanz and Aljama A: Vascular enumeration as a
prognosticator for colorectal carcinoma. Eur J Cancer. 36:55–60.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaio E, Tanaka S, Kitadai Y, Sumii M,
Yoshihara M, Haruma K and Chayama K: Clinical significance of
angiogenic factor expression at the deepest invasive site of
advanced colorectal carcinoma. Oncology. 64:61–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang JT, Huang KC, Jeng YM, Lee PH, Lai
HS and Hsu HC: Microvessel density, cyclo-oxygenase 2 expression,
K-ras mutation and p53 overexpression in colonic cancer. Br J Surg.
91:355–361. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng S, Han MY, Xiao ZX, Peng JP and Dong
Q: Clinical significance of vascular endothelial growth factor
expression and neovascularization in colorectal carcinoma. World J
Gastroenterol. 9:1227–1230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
White JD, Hewett PW, Kosuge D, McCulloch
T, Enholm BC, Carmichael J and Murray JC: Vascular endothelial
growth factor-D expression is an independent prognostic marker for
survival in colorectal carcinoma. Cancer Res. 62:1669–1675.
2002.PubMed/NCBI
|
|
29
|
Lindmark G, Gerdin B, Sundberg C, Pahlman
L, Bergström R and Glimelius B: Prognostic significance of the
microvascular count in colorectal cancer. J Clin Oncol. 14:461–466.
1996.PubMed/NCBI
|
|
30
|
Vermeulen P, Gasparini G, Fox SB, Toi M,
Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL
and Dirix LY: Quantification of angiogenesis in solid human
tumours: An international consensus on the methodology and criteria
of evaluation. Eur J Cancer. 32:2474–2484. 1996. View Article : Google Scholar
|
|
31
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
32
|
Pusztaszeri MP, Seelentag W and Bosman FT:
Immunohistochemical expression of endothelial markers CD31, CD34,
Von Willebrand factor, and Fli-1 in normal human tissues. J
Histochem Cytochem. 54:385–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duff SE, Li C, Garland JM and Kumar S:
CD105 is important for angiogenesis: Evidence and potential
applications. FASEB J. 17:984–992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Des Guetz G, Uzzan B, Nicolas P, Cucherat
M, Morere JF, Benamouzig R, Breau JL and Perret GY: Microvessel
density and VEGF expression are prognostic factors in colorectal
cancer. Meta-analysis of the literature. Br J Cancer. 94:1823–1832.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanigawa N, Amaya H, Matsumura M and
Shimomatsuya T: Correlation between expression of vascular
endothelial growth factor and tumor vascularity, and patient
outcome in human gastric carcinoma. J Clin Oncol. 15:826–832.
1997.PubMed/NCBI
|
|
36
|
Custodio A, Barriuso J, de Castro J,
Martínez-Marín V, Moreno V, Rodríguez-Salas N and Feliu J:
Molecular markers to predict outcome to antiangiogenic therapies in
colorectal cancer: Current evidence and future perspectives. Cancer
Treat Rev. 39:908–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kosaka Y, Mimori K, Fukagawa T, Ishikawa
K, Etoh T, Katai H, Sano T, Watanabe M, Sasako M and Mori M:
Identification of the high-risk group for metastasis of gastric
cancer cases by vascular endothelial growth factor receptor-1
overexpression in peripheral blood. Br J Cancer. 96:1723–1728.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
39
|
Okita NT, Yamada Y, Takahari D, Hirashima
Y, Matsubara J, Kato K, Hamaguchi T, Shirao K, Shimada Y, Taniguchi
H and Shimoda T: Vascular endothelial growth factor receptor
expression as a prognostic marker for survival in colorectal
cancer. Jpn J Clin Oncol. 39:595–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning Y, Lurje G, Danenberg K, Cooc J, Yang
D, Pohl A, Zhang W and Lenz H: VEGF and VEGFR1 gene expression
levels and tumor recurrence in adjuvant colon cancer. J Clin Oncol.
27:40402009.
|