Introduction
Polyacrylamide hydrogel (PAAG) is a jelly-like
transparent substance containing ~5% polyacrylamide and 95% water.
PAAG was first manufactured in Ukraine in the late 1980s and
introduced as a biomaterial for non-surgical breast augmentation.
The polyacrylamide in this compound was found to be non-toxic and
non-carcinogenic in several animal studies (1). PAAG injection for breast augmentation
has been used for ~10 years in certain Chinese medical facilities.
Although the clinical application of PAAG was banned on April 30,
2006 by the Chinese State Food and Drug Administration (2), the consequences and long-term
complications of this gel may not appear until several decades
later. The reported complications following PAAG injection for
breast augmentation include induration, pain, swelling, infection,
fever, aseptic inflammation, leakage, hematoma and gel migration
(3). Due to these complications, a
number of patients with mammoplasty augmentation history have
requested removal of the injected gel or a simultaneous second
augmentation as an alternative. However, in relation to the
development of malignant breast tumors following PAAG injection,
two cases of breast cancer occurring after injection of PAAG in
augmented breasts were reported in 2009 (4).
In this report, we present a case of malignant
breast tumor development following PAAG injection.
Case report
A 48-year-old woman underwent bilateral augmentation
mammoplasty using injectable PAAG in 2003. Following mammary
ptosis, the patient experienced hardening and a slow increase in
the size of the left breast. In February, 2013, the left breast
developed an infection, which was treated with antibiotic therapy
in another facility.
When the patient was admitted to our department for
further treatment in December, 2013, her vital signs were stable
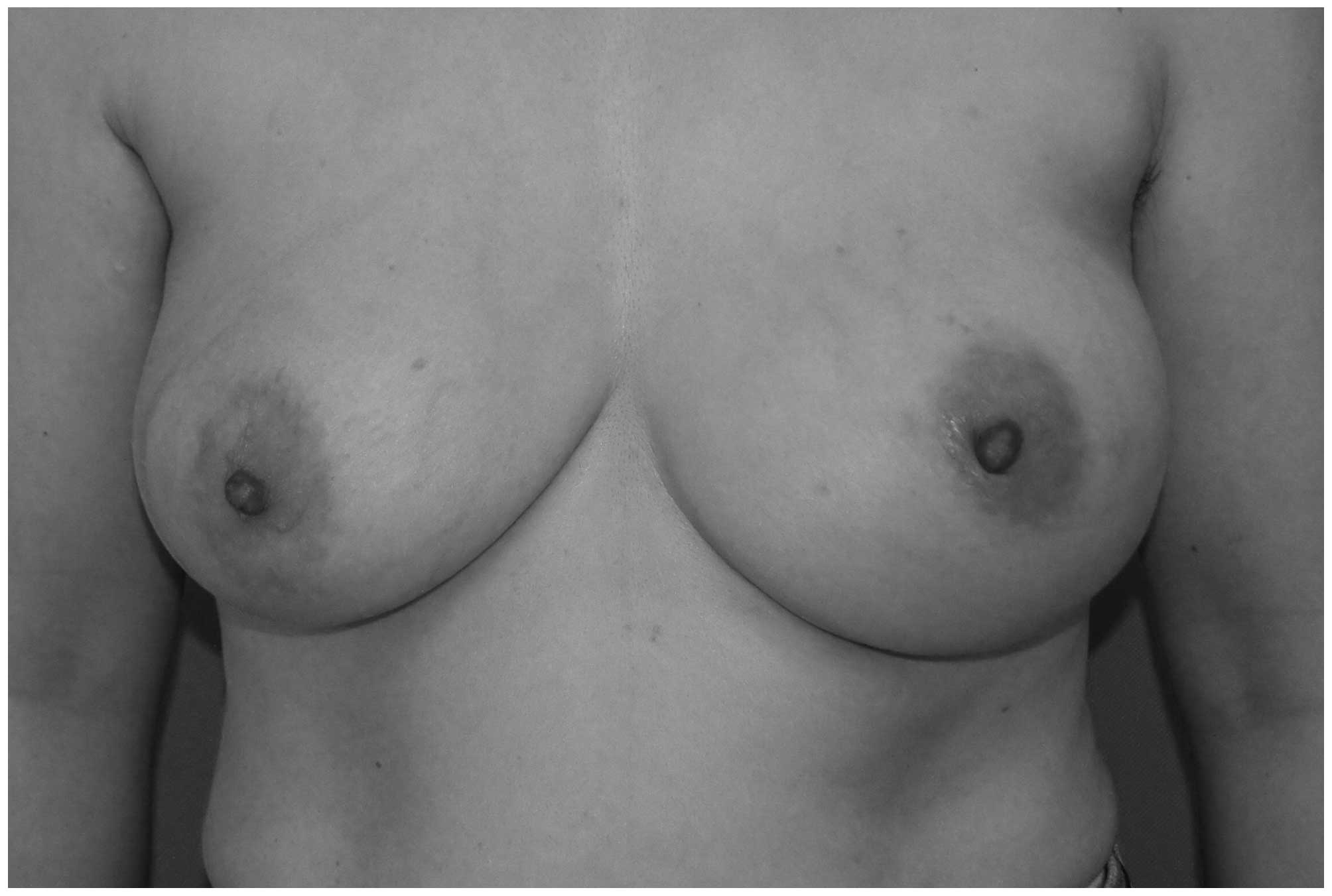
but her left breast appeared to be sagging, deformed and enlarged
compared with her right breast. The patient's areolar diameter
bilaterally was ~4.5 cm and the nipple diameter was ~1 cm. No
nipple discharge was observed (Fig.
1). On palpation, the left breast felt relatively harder, with
occasional pricking on compression. There was no palpable mass in
either breast. The blood biochemistry, six blood coagulation tests,
electrocardiography and chest radiography examinations were normal.
Magnetic resonance imaging (MRI) revealed post-injection augmented
breasts and abnormal signals of an indeterminate nature on the left
breast (Fig. 2). Although mammary
gland hyperplasia was considered possible, other diseases should be
excluded prior to diagnosis. The lymph nodes in the left axilla
were enlarged. Aspiration of the injected PAAG from both breasts
and biopsy of the inflamed mass in the left breast were performed.
Over 300 ml of injected PAAG were aspirated from each breast. The
histological examination revealed fibrous breast tissue, with a
silicon-like material.
Following surgery, an indurated mass appeared in the
patient's left breast, which continued to grow to the size of a
palm, without causing any discomfort. The patient was hospitalized
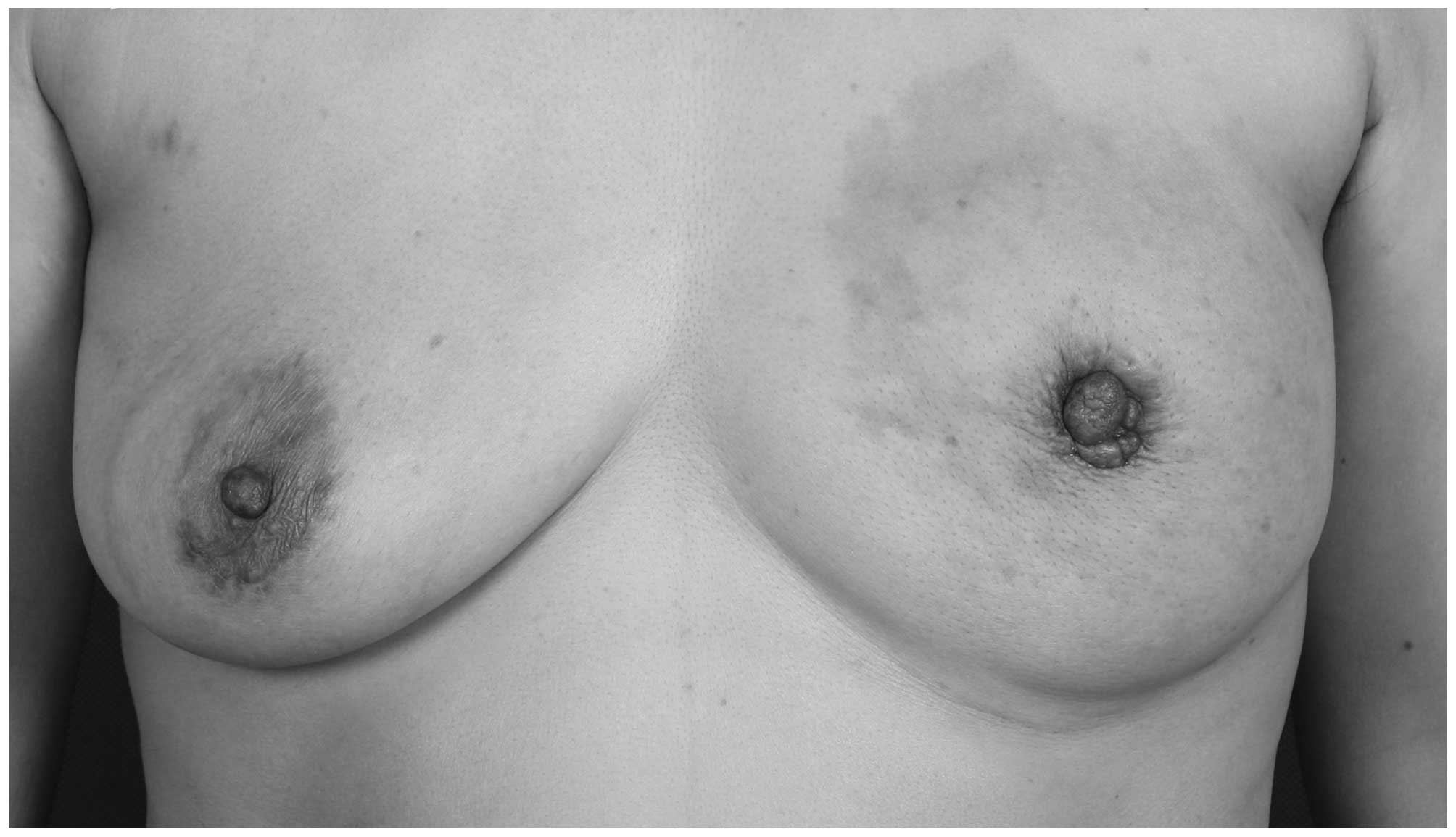
again in March, 2014 and her vital signs were stable. A 5-cm patch
of skin surrounding the left nipple appeared to be erythematous,
with fading of the color on compression. The skin in this area was
mildly puffy and pitted, resembling an orange peel. There were no
ulcers and the patient's skin temperature was normal (Fig. 3). On examination, a hard, painless,
ill-defined, fixed, 6×10-cm flaky mass was observed in the
patient's left breast, accompanied by a mild depression of the left
nipple. No regional enlarged lymph nodes were detected in either
axilla. The tumor was excised for histopathological examination and
the results revealed infiltration by stage II–III invasive breast
carcinoma (Fig. 4). The
immunohistological findings of the tumor were as follows: Estrogen
receptor (−), progesterone receptor (−), Cerb-B2 (++), local Ki-67
~60% (+), high-molecular weight CK (−), CK5/6 (−), scattered
calponin (+), P63 (−) and smooth muscle actin (+). The patient was
advised to undergo breast surgery and was referred to the oncology
department of the hospital for further treatment. No reccurence or
distant metastasis is reported to date.
Discussion
In this study, we present a case that demonstrates
the correlation between PAAG injection and breast cancer
development. Since breast augmentation by injection is considered
to be cosmetic surgery, pre-set control and randomized research
methods were not feasible in the present study.
A previous study has indicated that PAAG increases
the mRNA expression of the c-Myc proto-oncogene, which may inhibit
the growth and cause apoptosis of human fibroblasts and alter the
physical parameters of cells, such as their size and granularity
(5). This might increase the risk of
breast cancer, but further evidence is needed.
The patient was first hospitalized with a major
complaint of post-PAAG injection complications rather than the
occurrence of a tumor. During the first hospitalization, no typical
complaints, symptoms or signs of cancer (e.g., orange peel-like
skin; hard, ill-defined and fixed lump; or enlarged lymph nodes in
the axilla) were reported. Although certain signs and symptoms of a
breast tumor were observed during the patient's second
hospitalization, overlooking the possibility of cancer is possible
due to the limited number of case reports and studies on the
correlation between PAAG injection and breast cancer.
The currently available evidence indicate that
breast implants do not induce local or systemic disease,
particularly breast cancer (6–9). However,
implants may impair early-stage breast cancer identification by
mammography, as cosmetic breast implants are radio-opaque and
impair breast tissue visualization (8,10,11). Women with cosmetic breast implants may
present with late-stage tumors upon breast cancer diagnosis
(12).
Breast lump formation resulting from gel collection
in mammary tissues is the most common complication in PAAG-injected
augmentation mammoplasty patients. Compared with prosthetic
augmentation mammoplasty (PAM) patients with silicone implants,
early diagnosis of breast tumors is more difficult in PAAG-injected
patients, as differentiating between the injected hydrogel lump and
a possible neoplasm on palpation is very difficult.
Recent imaging techniques allow detection of breast
cancer in breasts with implants, even in cases without a palpable
mass (13). However, mammography
cannot accurately assess the postoperative status of PAAG-injected
breasts (14).
Ultrasonography and MRI are more efficient for
malignancy detection in PAM patients with implants compared with
mammography (15–19). Ultrasonography is the first step in
investigating symptomatic patients with augmented breasts aged
<40 years, in order to evaluate the breast or rule out
pathologies associated with the implant. This imaging technique is
also used as a complement to mammography in patients aged >40
years who present with pathological findings on screening or
diagnostic mammography (16,17).
MRI allows examination of breast tissue surrounding
the implant and exhibits a higher sensitivity compared with
mammography (18,19). Ultrasonography must be considered as a
routine adjunctive screening method for PAAG-injected patients.
When ultrasound detects abnormal pathologies associated with the
implant, MRI may be used as a highly reliable method for accurately
detecting masses in augmented breasts (20–22).
The results of our study are of great clinical
significance and suggest that physicians must be more aware of
possible breast cancer. A patient's medical history, physical
examination results and multiple imaging findings must be
comprehensively analyzed to avoid a misdiagnosis. More effective
methods must be established to visualize the lesions and
distinguish injected materials and inflamed masses from tumors.
During surgery, multiple tissue samples must be drawn from
suspicious nodules and sent for frozen section evaluation. No
significant difference in surgical intervention and prognosis is
reported between PAM and non-PAM patients with breast cancer
(23). However, based on the
patient's presentation, treatment and prognosis, more advanced
strategies must be designed for breast cancer patients with PAAG
injection mammoplasty.
PAAG injection augmentation may be correlated with
breast cancer, and breast cancer diagnosis in post-injection
augmentation mammoplasty patients may be difficult. While
ultrasonic and MRI methods have been used to detect breast cancer,
the sensitivity and accuracy of these techniques for distinguishing
between injected gel lumps and tumors must be improved to identify
tumors at the early stages of development. Great caution and
multiple methods may also be required to avoid misdiagnosis.
References
|
1
|
Amended final report on the safety
assessment of polyacrylamide and acrylamide residues in cosmetics.
Int J Toxicol. 24(Suppl 2): S21–S50. 2005.
|
|
2
|
State Food and Drug Administration: About
prohibited the use of polyacrylamide hydrogel (injection) warning.
http://www.sda.gov.cn/WS01/CL0493/93434.htmlAccessed.
May 10–2015
|
|
3
|
Luo SK, Chen GP, Sun ZS and Cheng NX: Our
strategy in complication management of augmentation mammaplasty
with polyacrylamide hydrogel injection in 235 patients. J Plast
Reconstr Aes. 64:731–737. 2011. View Article : Google Scholar
|
|
4
|
Cheng NX, Liu LG, Hui L, Chen YL and Xu
SL: Breast cancer following augmentation mammaplasty with
polyacrylamide hydrogel (PAAG) injection. Aesthetic Plast Surg.
33:563–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xi TF, Fan CX, Feng XM, Wan ZY, Wang CR
and Chou LL: Cytotoxicity and altered c-myc gene expression by
medical polyacrylamide hydrogel. J Biomed Mater Res A. 78:283–290.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deapen DM, Hirsch EM and Brody GS: Cancer
risk among Los Angeles women with cosmetic breast implants. Plast
Reconstr Surg. 119:1987–1992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deapen DM and Brody GS: Augmentation
mammaplasty and breast cancer: A 5-year update of the Los Angeles
study. Plast Reconstr Surg. 89:660–665. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoshaw SJ, Klein PJ, Clark BD, Cook RR and
Perkins LL: Breast implants and cancer: Causation, delayed
detection and survival. Plast Reconstr Surg. 107:1393–1408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinton LA, Lubin JH, Burich MC, Colton T,
Brown SL and Hoover RN: Breast cancer following augmentation
mammoplasty (United States). Cancer Causes Control. 11:819–827.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Handel N, Silverstein MJ, Gamagami P,
Jensen JA and Collins A: Factors affecting mammographic
visualization of the breast after augmentation mammaplasty. Jama.
268:1913–1917. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fajardo LL, Harvey JA, Mcaleese KA,
Roberts CC and Granstrom P: Breast-cancer diagnosis in women with
subglandular silicone gel-filled augmentation implants. Radiology.
194:859–862. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lavigne E, Holowaty EJ, Pan SY, Villeneuve
PJ, Johnson KC, Fergusson DA, Morrison H and Brisson J: Breast
cancer detection and survival among women with cosmetic breast
implants: Systematic review and meta-analysis of observational
studies. BMJ. 346:f23992013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark CP III, Peters GN and O'Brien KM:
Cancer in the augmented breast. Diagnosis and prognosis. Cancer.
72:2170–2174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng DHLM, Liu LG and Gong JS:
Mammographic appearance of postoperative augmentation mammoplasty
with hydrogel. Chin J Radiol. 40:354–356. 2006.
|
|
15
|
Leibman AJ and Kruse B: Breast cancer:
Mammographic and sonographic findings after augmentation
mammoplasty. Radiology. 174:195–198. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dhamanaskar KP, Muradali D, Kulkarni SR,
Bukhanov K, Pantazi SC and Wilson C: MRI directed ultrasound: A
cost-effective method for diagnosis and intervention in breast
imaging. Radiology. 225:653. 2002.
|
|
17
|
Esen G and Olgun DC: Ultrasonography of
the postsurgical breast including implants. Ultrasound Clinics.
3:295–329. 2008. View Article : Google Scholar
|
|
18
|
Juanpere S, Perez E, Huc O, Motos N, Pont
J and Pedraza S: Imaging of breast implants - a pictorial review.
Insights Imaging. 2:653–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orel SG and Schnall MD: MR imaging of the
breast for the detection, diagnosis and staging of breast cancer.
Radiology. 220:13–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung KC, Greenfield ML and Walters M:
Decision-analysis methodology in the work-up of women with
suspected silicone breast implant rupture. Plast Reconstr Surg.
102:689–695. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hold PM, Alam S, Pilbrow WJ, Kelly JF,
Everitt EM, Dhital SK and Juma A: How should we investigate breast
implant rupture? Breast J. 18:253–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng HL, Wu CC, Choi WM, Hui HS, Lu TN and
Chen LK: Breast cancer detection using magnetic resonance imaging
in breasts injected with liquid silicone. Plast Reconstr Surg.
104:2116–2120. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Handel N: The effect of silicone implants
on the diagnosis, prognosis and treatment of breast cancer. Plast
Reconstr Surg. 120(7 Suppl 1): S81–S93. 2007. View Article : Google Scholar
|