Introduction
Rhabdomyosarcoma (RMS) is a relatively uncommon
solid tumor in young patients, accounting for 4–8% of malignant
diseases in patients aged <15 years (1). It is reported that only 15–20% of the
RMS cases arise from the spermatic cord, epididymis, testicular
envelopes, prostate and bladder (2).
The clinical signs of paratesticular RMS are painless masses in the
inguinal canal or scrotum, displacing the testis without replacing
it. We herein report the case of a patient with epididymal
embryonal RMS, who presented with fever and enlargement of the
right epididymis, which was misdiagnosed as epididymitis. The
patient underwent 3 cycles of adjuvant chemotherapy postoperatively
and remains alive and recurrence-free after 4 years of
follow-up.
Case report
A 15-year-old adolescent presented to the outpatient
clinic of the Department of Andrology of the Norman Bethune First
Hospital (Changchun, China) with painful edema of the scrotum on
the right side that had lasted for 3 months. At the onset, the
patient complained that he had experienced a mechanical injury of
the scrotum while playing. The medical history of the patient was
unremarkable. The physical examination revealed an enlarged,
irregular, tender mass in the cauda of the epididymis, with a
diameter of 5 cm, and warm scrotal skin. The inguinal lymph nodes
were not palpable bilaterally. The abdomen was soft and non-tender,
without any positive findings. A scrotal ultrasound (Nemio XG 8 to
12 MHz linear array transducer; Toshiba, Tokyo, Japan) at
presentation revealed a hypoechoic swelling of the right epididymis
(54×26 mm) with marked increased vascularity, suspicious for severe
epididymitis. Therefore, the diagnosis of chronic epididymitis with
acute onset was made and antibiotics were administered by our
experienced physician.
After 3 weeks, the patient returned to the
department with severe pain and scrotal swelling; he was
hospitalized for further evaluation and was scheduled for an
exploratory surgery. The tumor markers α-fetoprotein, human
chorionic gonadotropin and serum lactate dehydrogenase were within
normal limits. During the operation, a hard, irregular tumor with
no abscess was identified on the cauda of the epididymis. An
intraoperative frozen-section biopsy revealed malignancy of the
epididymis. A complete radical right orchiectomy via the inguinal
approach and a right hemiscrotectomy were performed. An ipsilateral
inguinal lymph node was also removed for biopsy. A histological
examination of the surgical specimen demonstrated a spindle cell
variant, cytoplasmic eosinophils with atypical nuclei and
rhabdomyoblasts with eccentrically placed nuclei with prominent
nucleoli (Fig. 1). The tunica
albuginea had been invaded and the surgical margin was negative. On
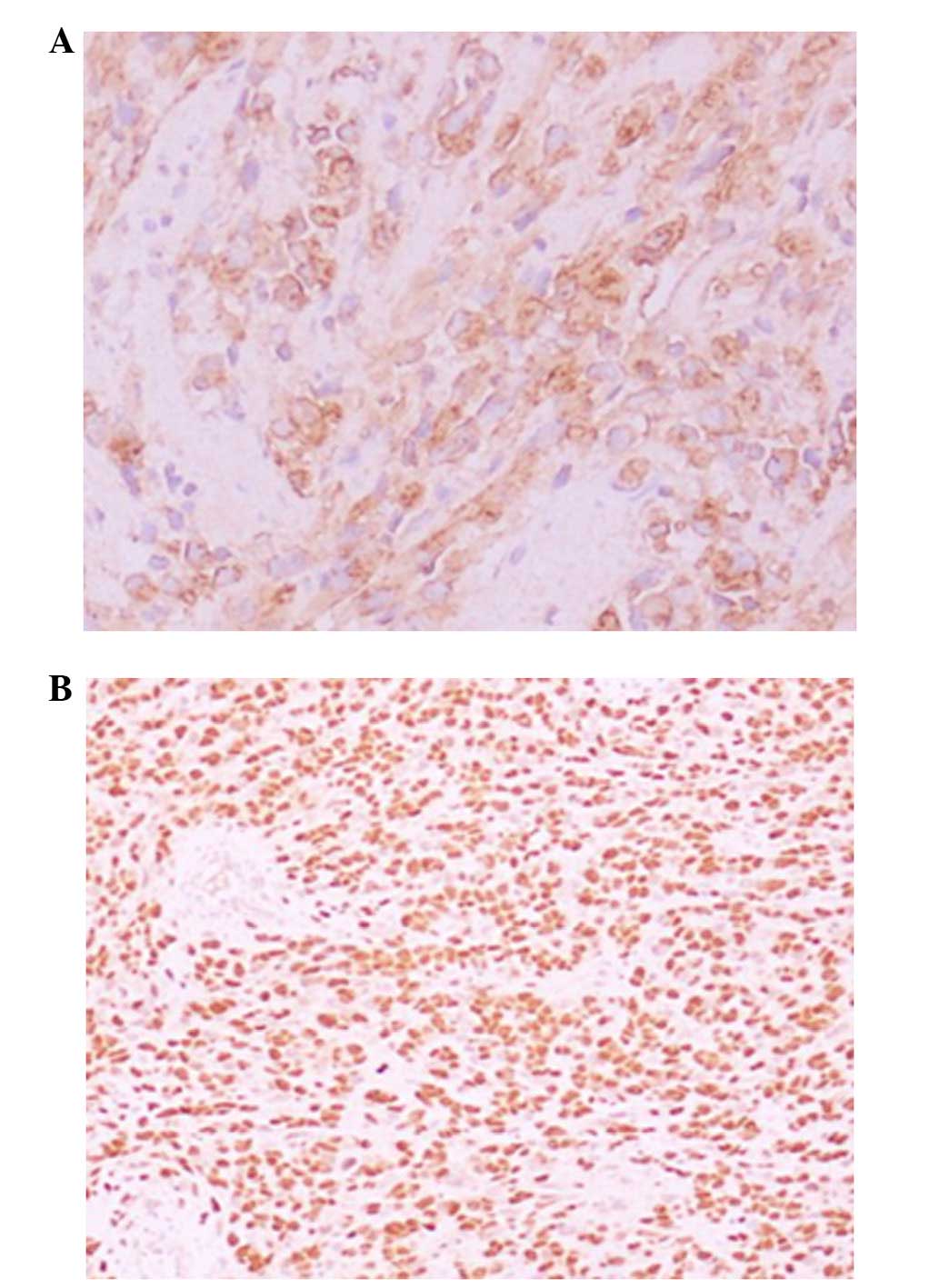
immunohistochemical examination, the tumor was found to be positive
for actin and myogenin and the diagnosis of RMS was confirmed
(Fig. 2). There was no evidence of
metastasis on postoperative thoracic and abdominal computed
tomography (CT) scans.
Following recovery, the patient received 3 cycles of
adjuvant chemotherapy with the VAC regimen (vincristine 1.5
mg/m2, actinomycin 1.5 mg/m2 and
cyclophosphamide 500 mg/m2), and was followed closely.
After a 4-year follow-up, the patient remained in good condition,
with no evidence of recurrence. No metastasis was detected during
this period.
The patient and his family consented to the
publication of the clinical data.
Discussion
Paratesticular RMSs, which comprise 7% of all RMSs,
are rare tumors with an aggressive growth pattern that belong to
the same family of malignancies derived from primitive mesenchymal
cells, such as Ewing's sarcoma, and may be related. It has been
reported that the ages of 4 and 18 years represent two frequency
peaks for the development of RMS (3).
The most common histological types of RMS, according to the
international classification of RMS, are embryonal, alveolar,
botryoid embryonal, spindle cell embryonal and anaplastic (4). Our patient had an embryonal RMS, which
is very rare, with only few cases reported in the literature.
The typical patient presentation for paratesticular
RMS is a painless scrotal mass, or symptoms of metastasis, such as
inguinal lymphadenopathy, fatigue, decreased appetite and weight
loss. When patients present with a painful, edematous scrotum, they
are often misdiagnosed with epididymitis. Pain has been reported in
only 7% of the cases (5), whereas
edema is more frequent, and a hydrocele may be occasionally present
(6). Kim et al described a
case of paratesticular RMS accompanied by epididymitis manifesting
as painful scrotal edema (7). In our
case, an edematous, painful scrotum and warm scrotal skin were the
initial symptoms. Moreover, the patient recalled a history of
scrotal injury. As a result, the diagnosis of malignancy was
excluded. Even at the second visit, epididymitis and epididymal
abscess were strongly suspected. The gradual enlargement of the
testis and persistent pain, not relieved by antibiotics, prompted
us to perform an exploratory surgery, during which the epididymal
mass associated with epididymitis was identified. Therefore, the
RMS in our patient was present concomitantly with epididymitis,
which masked the primary lesion.
Scrotal sonography is the initial imaging modality
for the evaluation of a scrotal mass. This imaging modality shows a
mass with heterogeneous echogenicity and inguinoscrotal extension
in 80% of the cases (8). Wood and
Dewbury reported a case in which ultrasonography revealed increased
epididymal and testicular blood flow, consistent with
epididymo-orchitis (9). A
thoraco-abdomino-pelvic CT scan is usually performed to evaluate
lymph node and distant metastases. However, the diagnosis is
confirmed using histology. The histological appearance of embryonal
RMS is characterized by the presence of undifferentiated
patternless spindled cells and small round blue cells.
Immunohistochemical markers are crucial for the differential
diagnosis of RMS from other primary mesenchymal and germ cell
tumors also exhibiting rhabdomyoblastic differentiation (10). Markers such as myo-D1 and myoglobin
are more specific compared with muscle-specific actin, smooth
muscle actin and desmin, as smooth muscle markers are of limited
value in differential diagnosis. The more mature muscle cells stain
strongest for myoglobin, smooth muscle actin and desmin, whereas
immature embryonal skeletal muscle cells stain strongest for
vimentin (11). Our
immunohistochemical stains were strongly and diffusely positive for
actin and myogenin, suggesting a malignant mesenchymal tumor with
rhabdomyoblast differentiation. Electron microscopy is considered
to be of value in the diagnosis of RMS, as it demonstrates networks
of myosin filaments lined by free ribosomes (12).
The management of embryonal RMS involves a
multimodal approach. Complete surgical debulking followed by
adjuvant chemotherapy is currently considered to be the treatment
of choice. In cases who have previously undergone transscrotal
surgery or if the tumor is fixed to the scrotal wall, inguinal
orchiectomy and hemiscrotectomy should be performed, including
radical excision of the scrotal skin (13). There has been significant controversy
regarding the importance of performing a systematic
lymphadenectomy, as 19–38% of the tumors present with lymph node
involvement at diagnosis. Abhijith et al reported that
patients aged >10 years, with or without radiographic evidence
of retroperitoneal disease, should undergo a staging
retroperitoneal lymph node dissection and receive radiation in
addition to chemotherapy if the lymph nodes are positive (14). The current tendency is to avoid
dissection if the radiological examinations reveal no lymph nodes
sized >1 cm (15). RMS is a
chemosensitive tumor, and the role of multiagent chemotherapy in
its treatment has been clearly demonstrated. Several
chemotherapeutic protocols have been applied. The VAC, IVA and VIE
protocols (E, etoposide and I, ifosfamide) were mainly used and
superior results were observed with the VAC protocol (16).
The prognosis and optimal management of adult
embryonic RMS is uncertain, due to its rarity. Prognosis is
generally better if the tumor is confined to the scrotum and when
found in younger patients, whereas adult onset of RMS,
retroperitoneal location and alveolar histology are all associated
with a poor prognosis (17).
Combinations of polychemotherapy, surgery and radiotherapy have
yielded survival rates as high as 85% in patients with localized
disease (18). In the present case,
visible tumor growth and tunica albuginea invasion on the final
pathological examination were considered to be indications for
multimodality therapy. The edema of the epididymis in our patient
neither resulted in the development of embryonic RMS, nor delayed
the administration of the treatment. On the contrary, this unique
symptom prompted the patient to seek treatment and early
intervention yielded a better prognosis.
This case was illustrative of several points worthy
of notice: The clinical presentation was unusual, which made the
tumor difficult to diagnose preoperatively. The location and
rapidly progressive course of the tumor were typical of embryonal
RMS and it may be possible to obtain successful results with
chemotherapy protocols in these tumors. Considering its tendency to
metastasise, long-term periodic surveillance is warranted.
References
|
1
|
Weiss AR, Lyden ER, Anderson JR, Hawkins
DS, Spunt SL, Walterhouse DO, Wolden SL, Parham DM, Rodeberg DA,
Kao SC and Womer RB: Histologic and clinical characteristics can
guide staging evaluations for children and adolescents with
rhabdomyosarcoma: A report from the Children's Oncology Group Soft
Tissue Sarcoma Committee. J Clin Oncol. 31:3226–3232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmed HU, Arya M, Muneer A, Mushtaq I and
Sebire NJ: Testicular and paratesticular tumours in the prepubertal
population. Lancet Oncol. 11:476–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bouchikhi AA, Mellas S, Tazi MF, Lahlaidi
K, Kharbach Y, Benhayoune K, Kanab R, Elammari JE, Khallouk A, El
Fassi MJ and Farih MH: Embryonic paratesticular rhabdomyosarcoma: A
case report. J Med Case Rep. 7:932013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qualman S, Lynch J, Bridge J, Parham D,
Teot L, Meyer W and Pappo A: Prevalence and clinical impact of
anaplasia in childhood rhabdomyosarcoma: A report from the Soft
Tissue Sarcoma Committee of the Children's Oncology Group. Cancer.
113:3242–3247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khoubehi B, Mishra V, Ali M, Motiwala H
and Karim O: Adult paratesticular tumours. BJU Int. 90:707–715.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaslau S, Perlmutter AE, Farivar-Mohseni
H, Chang WW and Kandzari SJ: Rhabdomyosarcoma of tunica vaginalis
masquerading as hydrocele. Urology. 65:10012005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YJ, Huh JS, Hyun CL and Kim SD: A case
of pediatric paratesticular rhabdomyosarcoma with epididymitis.
World J Mens Health. 30:146–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiener ES, Lawrence W, Hays D, Lobe TE,
Andrassy R, Donaldson S, Crist W, Newton W, Johnson J, Gehan E, et
al: Retroperitoneal node biopsy in paratesticular rhabdomyosarcoma.
J Pediatr Surg. 29:171–177; discussion 178. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood A and Dewbury KC: Case report:
Paratesticular rhabdomyosarcoma - colour Doppler appearances. Clin
Radiol. 50:130–131. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kahn DG: Rhabdomyosarcoma mimicking acute
leukemia in an adult: Report of a case with histologic, flow
cytometric, cytogenetic, immunohistochemical, and ultrastructural
studies. Arch Pathol Lab Med. 122:375–378. 1998.PubMed/NCBI
|
|
11
|
Hardaway CA, Graham BS, Barnette DJ and
Feldman BD: Embryonal rhabdomyosarcoma presenting in an adult: A
case report and discussion of immunohistochemical staining. Am J
Dermatopathol. 25:45–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erlandso RA: The ultrastructural
distinction between rhabdomyosarcoma and other undifferentiated
‘sarcomas’. Ultrastruct Pathol. 11:83–101. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung JM, Lim YT and Lee SD: Infantile
testicular rhabdomyosarcoma. Urology. 69:1208.e13–e15. 2007.
View Article : Google Scholar
|
|
14
|
Abhijith SM, Nerli RB, Weiss D and
Srinivasan A: Lararoscopic retroperitoneal lymph node dissection
for paratesticular rhabdomyosarcoma in older children/adolescents.
Indian J Surg Oncol. 4:341–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahsaini M, Kassogué A, Tazi MF, Idrissi
KS, Moumna K and Chbani L: Early local recurrence of a pure
embryonal rhabdomyosarcoma of the testis successfully managed with
local resection associated with lymphadenectomy and adjuvant
chemotherapy. J Afr Cancer. 6:115–118. 2014. View Article : Google Scholar
|
|
16
|
Kourda N, El Atat R, Derouiche A, Bettaib
I, Baltagi S and Zermani R: Paratesticular pleomorphic
rhabdomyosarcoma in an adult: Diagnosis and management. Cancer
Radiother. 11:280–283. 2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tay YK, Morelli JG, Weston WL, Stork LC
and Ruyle SZ: Congenital subcutaneous nodule in an infant. Pediatr
Dermatol. 15:403–405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrari A, Bisogno G, Casanova M, Meazza
C, Piva L, Cecchetto G, Zanetti I, Pilz T, Mattke A, Treuner J and
Carli M: Paratesticular rhabdomyosarcoma: Report from the Italian
and German Cooperative Group. J Clin Oncol. 20:449–455. 2002.
View Article : Google Scholar : PubMed/NCBI
|