Introduction
Renal cell carcinoma (RCC), which accounts for ~5%
of all epithelial cancer types, is the ninth most common cancer
worldwide. Around 20% of patients experience recurrence or develop
metastatic RCC following nephrectomy (1). Although late recurrence of RCC following
curative initial surgery is not a rare event, a previous study
demonstrated that contralateral adrenal metastasis of RCC is rare
(2). Endoscopic ultrasound-guided
fine-needle aspiration (EUS-FNA) is a relatively novel modality for
obtaining samples from deep-seated lesions. In a previous study,
adrenal gland samples obtained by EUS-FNA biopsy were adequate for
determining a pathological diagnosis (3). The present study reported a rare case of
contralateral adrenal metastasis of RCC, which was diagnosed by
EUS-FNA.
Case report
A 76-year-old female with a complaint of cognitive
dysfunction visited the Department of Neurosurgery, Kanazawa
University Hospital (Ishikawa, Japan). The patient was previously
diagnosed with RCC of the right kidney in the Department of
Urology, Public Central Hospital of Matto (Ishikawa, Japan) 19
years previously. The right kidney was removed and the patient
received no adjuvant therapy at that time. A physical examination
revealed no findings other than disorientation, and laboratory
data, including tumor markers, revealed no notable findings. Brain
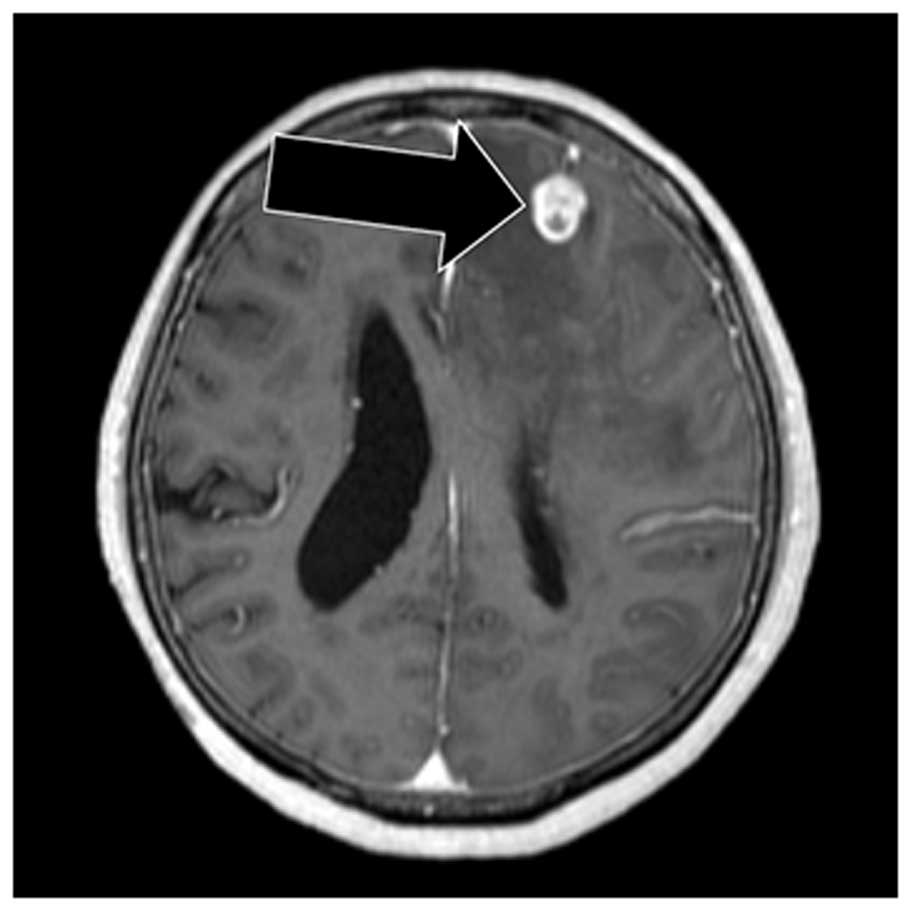
gadolinium contrast-enhanced magnetic resonance imaging uncovered
several nodules (Fig. 1), whereas
chest and abdominal computed tomography (CT) identified a single
nodule in the right lower lobe of the lungs, a mass in the left
adrenal gland and an osteolytic lesion in the left pelvis (Data not
shown). On the basis of these findings, the patient was diagnosed
with a metastatic brain tumor. Following diagnosis, the patient
received stereotactic radiosurgery with gamma knife therapy for the
brain lesions. To identify the primary lesion, the present case
study attempted to obtain histological confirmation of the adrenal
lesion using EUS-FNA, available in the department. FNA was
performed via the transgastric approach with linear EUS (GF-UCT260;
Olympus, Tokyo, Japan), and two passes were made with a 19-gauge
needle (Sono Tip Pro Control; Medi-Globe, Rosenheim, Germany). EUS
revealed a homogeneous hypoechoic mass with a maximum diameter of
26 mm within the left adrenal gland (Fig.
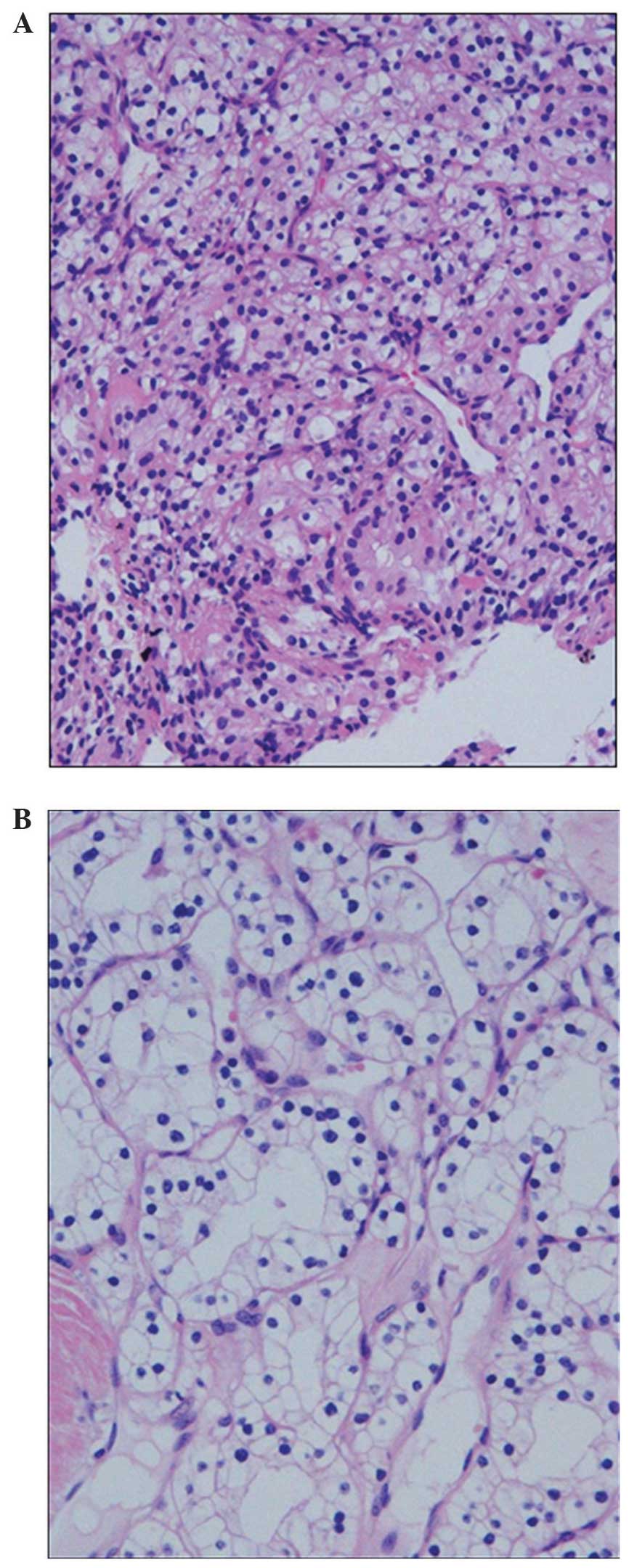
2). Tissue obtained from the aspirated material revealed clear
cytoplasmic and vascular stroma (Fig.
3A), and these findings were similar to those of the
nephrectomy specimen obtained 19 years previously (Fig. 3B). Histologically, a definitive
diagnosis of metastatic RCC was made. Based on the diagnosis, the
patient received targeted therapy with pazopanib (Votrient®;
GlaxoSmithKline, Middlesex, UK; 800 mg orally, once/day). The
majority of the metastatic lesions of RCC regressed following 1
month of treatment (Fig. 4A–C).
Discussion
RCC, accounting for 2–3% of all cancer types, is the
most common cause of mortality concerning urologic malignancies
(4), and it is more common in males
compared with females. RCC generally occurs between 50 and 70 years
of age, however, it can occur at any age. Risk factors for RCC are
family history, including von Hippel-Lindau (VHL) disease and
Birt-Hogg-Dubé syndrome, cigarette smoking, obesity, and
hypertension. Concerning family history, an inherited
predisposition to RCC accounts for only 3–4% of all cases. By
contrast, the majority of RCCs occur sporadically (5). Histologically, RCC is classified on the
basis of the tissue type. In particular, clear cell RCC accounts
for ~80% of all RCCs, whereas papillary (<15%), chromophobe
(<5%) and collecting duct carcinomas (<1%) comprise the
remaining cases. Several previous studies confirmed that 30–60% of
sporadic clear cell RCCs express mutations in VHL, which
consequently leads to high expression of vascular endothelial
growth factor (VEGF), transforming growth factor α, glucose
transporter-1 and carbonic anhydrase 9, associated with arborizing
vasculature and abundant cytoplasm (6,7). It was
determined that ~75% of patients with RCC are diagnosed with
localized disease, and surgical resection has been the gold
standard for treating localized RCC. In addition, it is evident
from two previous randomized phase III studies that cytoreductive
nephrectomy prior to systemic treatment improves the survival rate
for patients with metastatic RCC (8,9). By
contrast, systemic treatment of metastatic RCC has markedly changed
over the last decade as a result of the development of targeted
therapies and immunotherapies (1).
Although ~60% of cases of recurrent RCC following
nephrectomy for localized disease occur within 12 months, late
recurrence of RCC beyond 10 years, as noted in the present study,
is unusual. According to a previous study performed by Nakano et
al (10) and Miyao et al
(11), ~5% of patients who were
disease-free for 10 years following nephrectomy developed late
recurrence of RCC. Furthermore, Miyao et al (11) noted that lymph node metastasis was
predictive of late recurrence. Conversely, lymph node metastasis
was not observed in the present patient at the initial surgery.
Metastasis of RCC can occur at any organ, including
the lungs, kidneys, bone, brain, liver and adrenal gland. The
contralateral adrenal grand, which was biopsied in the patient, was
a rare site of metastasis of RCC, being detected in 2.5% of
patients with metastatic RCC at autopsy (2). The spread of RCC to the contralateral
adrenal gland in the present case may not have been a recent event
since a previous study by Lau et al (12) reported that the mean interval to
developing contralateral adrenal metastasis following radical
nephrectomy is 5.2 years (12).
EUS-FNA was developed in 1992 and has been widely
used for diagnosing perigastrointestinal lesions. The diagnostic
capacity of EUS-FNA for adrenal lesions has been less investigated
in comparison with that of pancreatic lesions, for which the
sensitivity and specificity were reported as 78–95 and 75–100%,
respectively (13). A transgastric
approach of EUS-FNA can provide proximity to the left adrenal gland
compared with traditional percutaneous techniques, including
CT-guided FNA, significantly reducing the risk of complications
(14). Additionally, real-time
ultrasound-guided needling with color Doppler guidance enables the
avoidance of vascular structures, and therefore, EUS-FNA decreases
the risk of bleeding. Considering the advantages of EUS-FNA, the
modality is applicable for diagnosing adrenal metastases of unknown
primary tumors, as observed in the present case. However, FNA
biopsy of adrenal pheochromocytoma can induce fatal hypertensive
crisis. Therefore, EUS-FNA of adrenal lesions is best performed if
the possibility of pheochromocytoma has been eliminated. Regarding
the patient, the success in obtaining adrenal gland tissue using
EUS-FNA prevented unnecessary diagnostic surgeries.
Targeted therapies, including targeting the VEGF
receptor and the mechanistic targeting of rapamycin inhibitors, for
metastatic RCC have been previously developed (1). With this development, progression-free
survival (PFS) for patients with metastatic RCC has been markedly
prolonged. The first-line treatment for the present patient was
pazopanib, which proved to be non-inferior to sunitinib with
respect to PFS. Pazopanib was selected as the treatment modality,
as a result of its superior safety and quality-of-life profiles
compared with sunitinib (15). It was
determined that pazopanib is an appropriate treatment modality for
patients with metastatic RCC who have mild cognitive impairment,
including the present patient, as these patients may have
difficulty informing caregivers about adverse events.
Progress in the diagnosis of rare cases of
contralateral adrenal metastasis from RCC indicates that EUS-FNA of
adrenal metastases of unknown primary origin is available and
beneficial from the perspective of invasiveness.
References
|
1
|
Jonasch E, Gao J and Rathmell WK: Renal
cell carcinoma. BMJ. 349:g47972014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saitoh H, Nakayama M, Nakamura K and Satoh
T: Distant metastasis of renal adenocarcinoma in nephrectomized
cases. J Urol. 127:1092–1095. 1982.PubMed/NCBI
|
|
3
|
Jhala NC, Jhala D, Eloubeidi MA, Chhieng
DC, Crowe DR, Roberson J and Eltoum I: Endoscopic ultrasound-guided
fine-needle aspiration biopsy of the adrenal glands: Analysis of 24
patients. Cancer. 102:308–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chin AI, Lam JS, Figlin RA and Belldegrun
AS: Surveillance strategies for renal cell carcinoma patients
following nephrectomy. Rev Urol. 8:1–7. 2006.PubMed/NCBI
|
|
5
|
Lipworth L, Tarone RE, Lund L and
McLaughlin JK: Epidemiologic characteristics and risk factors for
renal cell cancer. Clin Epidemiol. 1:33–43. 2009.PubMed/NCBI
|
|
6
|
Shiao YH, Forsti A, Egevad L, Anderson LM,
Lindblad P and Hemminki K: VHL down-regulation and differential
localization as mechanisms in tumorigenesis. Kidney Int.
64:1671–1674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei
MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al: Mutations of the
VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7:85–90.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mickisch GH, Garin A, van Poppel H, de
Prijck L and Sylvester R: European Organisation for Research and
Treatment of Cancer (EORTC) Genitourinary Group. Radical
nephrectomy plus interferon-alfa-based immunotherapy compared with
interferon alfa alone in metastatic renal-cell carcinoma: A
randomised trial. Lancet. 358:966–970. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flanigan RC, Salmon SE, Blumenstein BA,
Bearman SI, Roy V, McGrath PC, Caton JR Jr, Munshi N and Crawford
ED: Nephrectomy followed by interferon alfa-2b compared with
interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J
Med. 345:1655–1659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakano E, Fujioka H, Matsuda M, Osafune M,
Takaha M and Sonoda T: Late recurrence of renal cell carcinoma
after nephrectomy. Eur Urol. 10:347–349. 1984.PubMed/NCBI
|
|
11
|
Miyao N, Naito S, Ozono S, Shinohara N,
Masumori N, Igarashi T, Nakao M, Tsushima T, Senga Y, Horie S, et
al: Late recurrence of renal cell carcinoma: Retrospective and
collaborative study of the Japanese society of renal cancer.
Urology. 77:379–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau WK, Zincke H, Lohse CM, Cheville JC,
Weaver AL and Blute ML: Contralateral adrenal metastasis of renal
cell carcinoma: Treatment, outcome and a review. BJU Int.
91:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshinaga S, Suzuki H, Oda I and Saito Y:
Role of endoscopic ultrasound-guided fine needle aspiration
(EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc.
23(Suppl 1): S29–S33. 2011. View Article : Google Scholar
|
|
14
|
Ang TL, Chua TS, Fock KM, Tee AK, Teo EK
and Mancer K: EUS-FNA of the left adrenal gland is safe and useful.
Ann Acad Med Singapore. 36:954–957. 2007.PubMed/NCBI
|
|
15
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|