Introduction
Pleomorphic carcinoma of the lung is a rare, highly
malignant tumor, accounting for 0.1–0.4% of primary lung cancer
cases (1–3). It has a more aggressive clinical course
compared with other types of non-small-cell lung cancer (NSCLC)
(4–6),
as well as a poorer outcome (4,7,8). We herein report two cases of pleomorphic
carcinoma of the lung, who were treated with pemetrexed-containing
chemotherapy. Our results demonstrated that pemetrexed-containing
chemotherapy may be key to the treatment of advanced cases with
this type of lung cancer.
Case report
Case 1
A 68-year-old man was admitted to the hospital with
a productive cough. A chest radiograph revealed a tumor 5 cm in
diameter in the right lung. Bioptic specimens, which were obtained
transbronchially, were diagnosed as NSCLC. As the patient did not
present with hilar/mediastinal lymph node enlargement or distant
metastasis, he underwent right upper pulmonary lobectomy. On
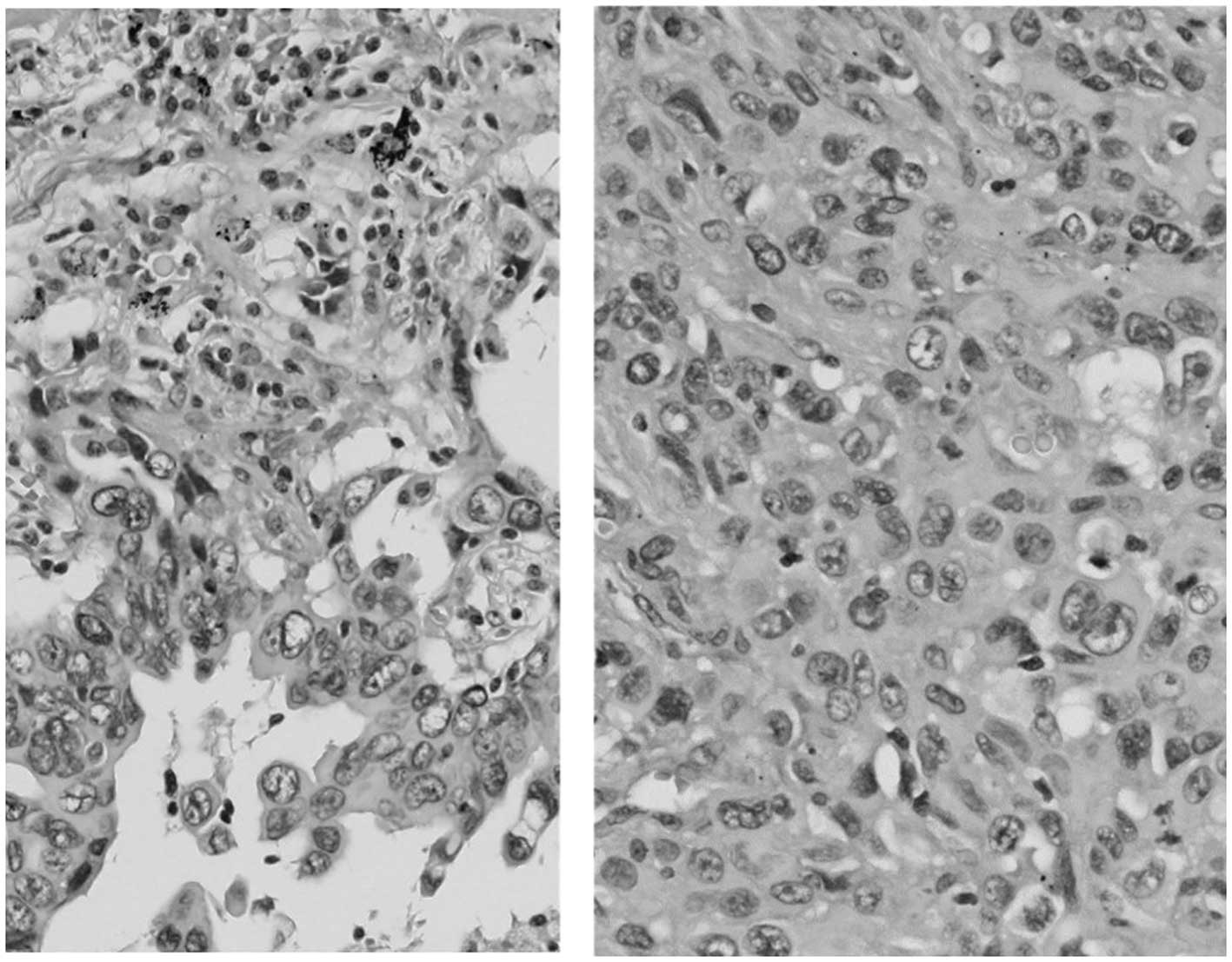
postoperative pathological examination, the tumor was diagnosed as
pleomorphic carcinoma of the lung (Fig.
1). The examination for epidermal growth factor receptor
mutations was negative. As there was microscopic involvement of the
ipsilateral mediastinal lymph nodes, 4 courses of postoperative
chemotherapy containing cisplatin and vinorelbine were
administered. Four months after the end of the treatment (10 months
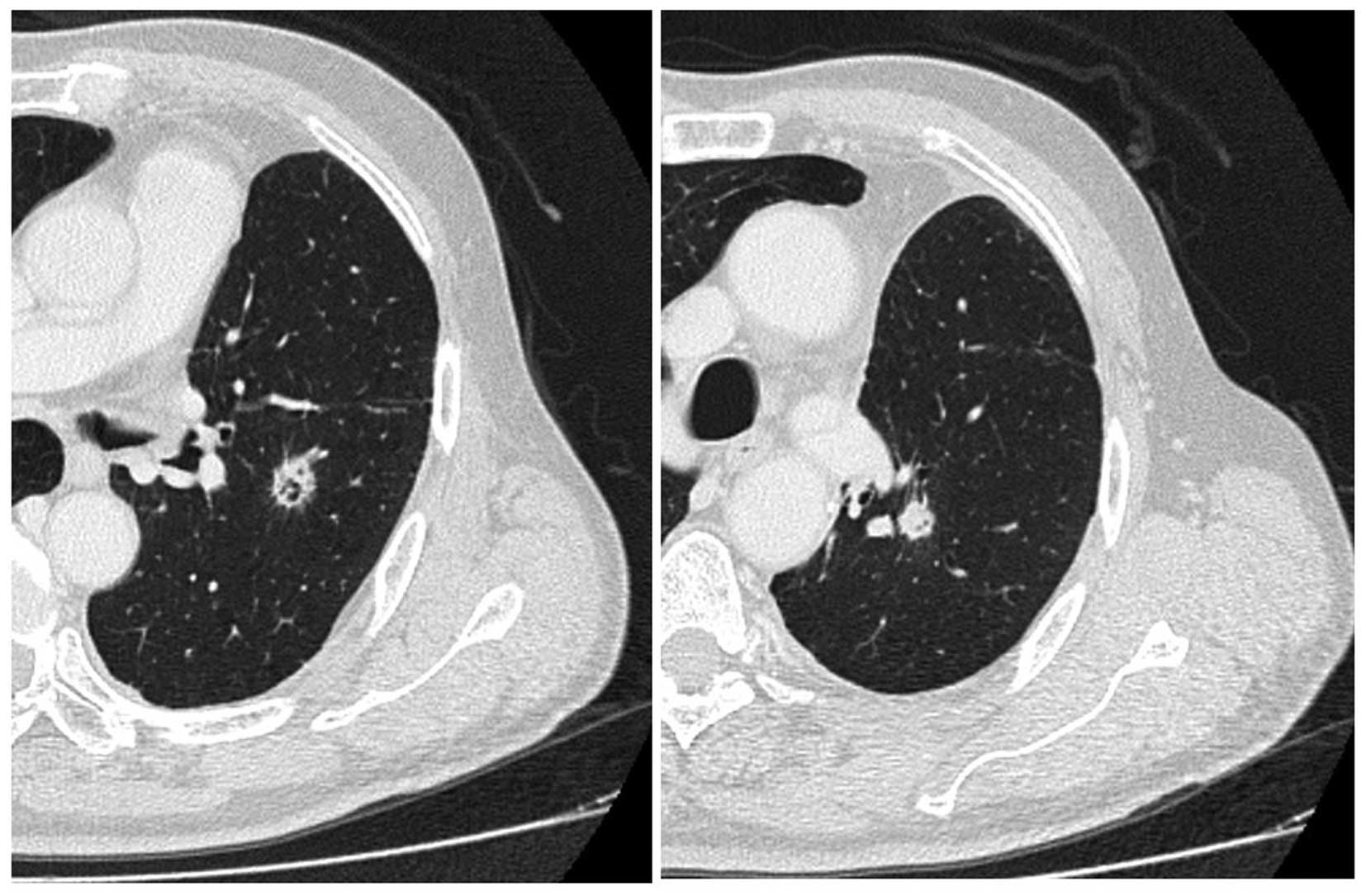
after the diagnosis), the disease recurred as two pulmonary nodules
≤1 cm in diameter in the left lung, detected on follow-up chest
computed tomography (CT) scan (Fig.
2). The work-up for systemic metastasis was negative. Two
courses of chemotherapy, consisting of carboplatin [area under the
curve (AUC) = 5, day 1, q28 days), pemetrexed (500
mg/m2, day 1, q28 days) and bevacizumab (15 mg/kg, day
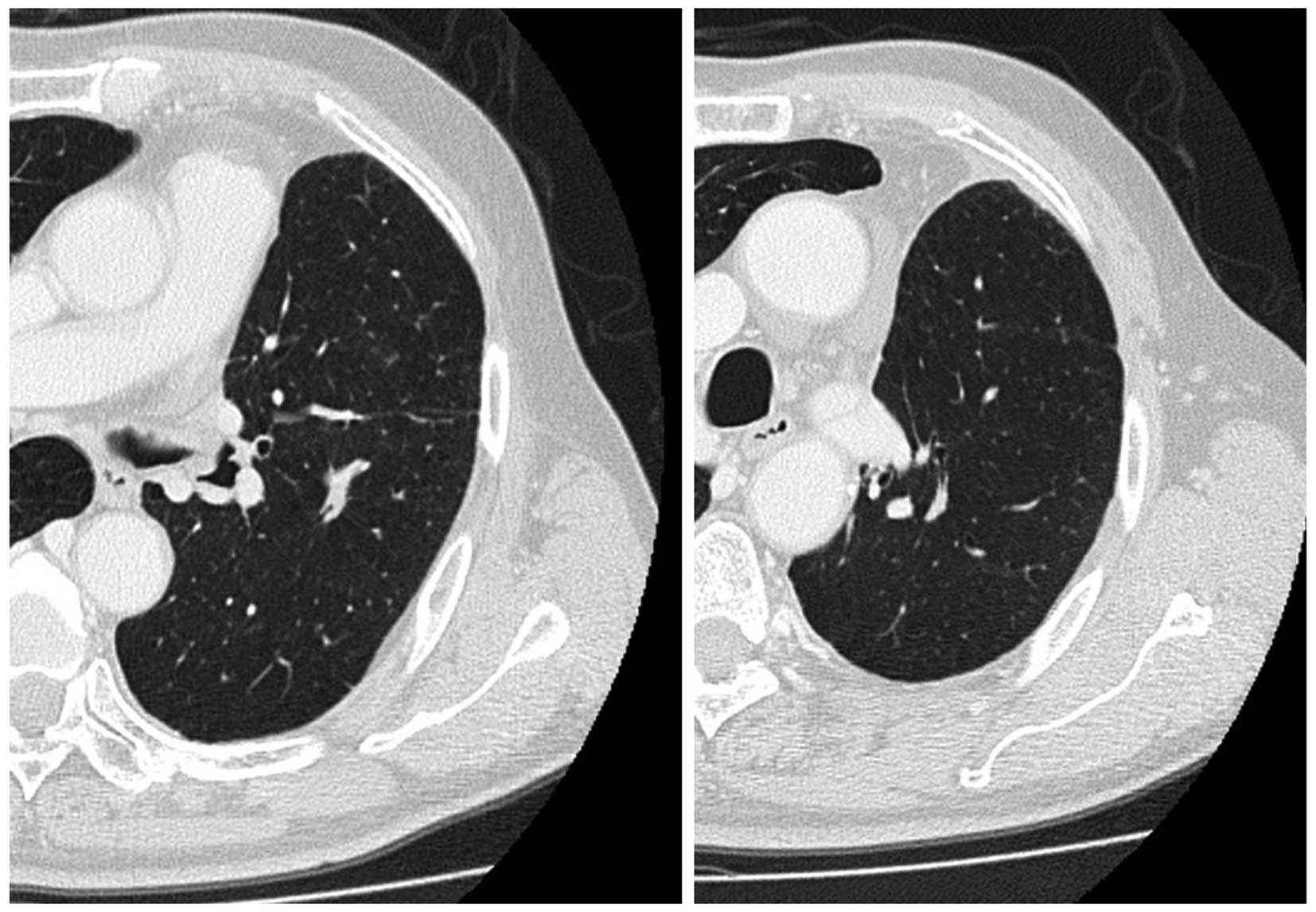
1, q28 days) was performed, and the post-treatment evaluation CT
scan revealed shrinkage of the two pulmonary lesions (Fig. 3). Thereafter, the patient received an
additional two courses of chemotherapy. A follow-up CT scan
revealed further shrinkage of the pulmonary tumors. The patient
received 20 courses of maintenance chemotherapy consisting of
pemetrexed (500 mg/m2, day 1, q28 days) and bevacizumab
(15 mg/kg, day 1, q28 days) without any severe adverse events. No
recurrence was found and the patient remained well for 30 months
after the initiation of the chemotherapy for recurrence; however,
the patient succumbed to brain infarction.
Case 2
A 46-year-old man was admitted to the hospital with
anorexia, fever and weight loss. The blood tests revealed a white
blood cell count (WBC) of 22,900/µl with 78% neutrophils, a red
blood cell count of 399×104/µl, and a platelet count of
43.2×104/µl. The patient's hemoglobin concentration was
11.5 g/dl and the hematocrit was 34.8%. On the blood chemistry
tests, the serum albumin was 2.9 mg/dl, the alkaline phosphatase
348 U/l, the total bilirubin 0.3 mg/dl, and the creatinine 0.57
mg/dl. The serum transaminase levels were within normal limits, and
blood serology revealed a C-reactive protein (CRP) level of 15.64
mg/dl. The granulocyte/colony-stimulating factor (G-CSF) level was
246 U/l (normal, ≤39 U/l). A sizeable mass was identified in the
upper lobe of the right lung by chest radiography (Fig. 4). A biopsy specimen obtained by
video-assisted thoracoscopic surgery revealed a poorly
differentiated lung carcinoma. The patient received 4 courses of
chemotherapy with carboplatin (AUC=5, day 1, q28 days), pemetrexed
(500 mg/m2, day 1, q28 days) and bevacizumab (15 mg/kg,
day 1, q28 days), followed by chemoradiotherapy (60 Gy). WBC
decreased to within the normal range and fever subsided tentatively
for several weeks after each course of chemotherapy. The response
was evaluated as ‘stable disease’.
Six months after the initiation of the chemotherapy,
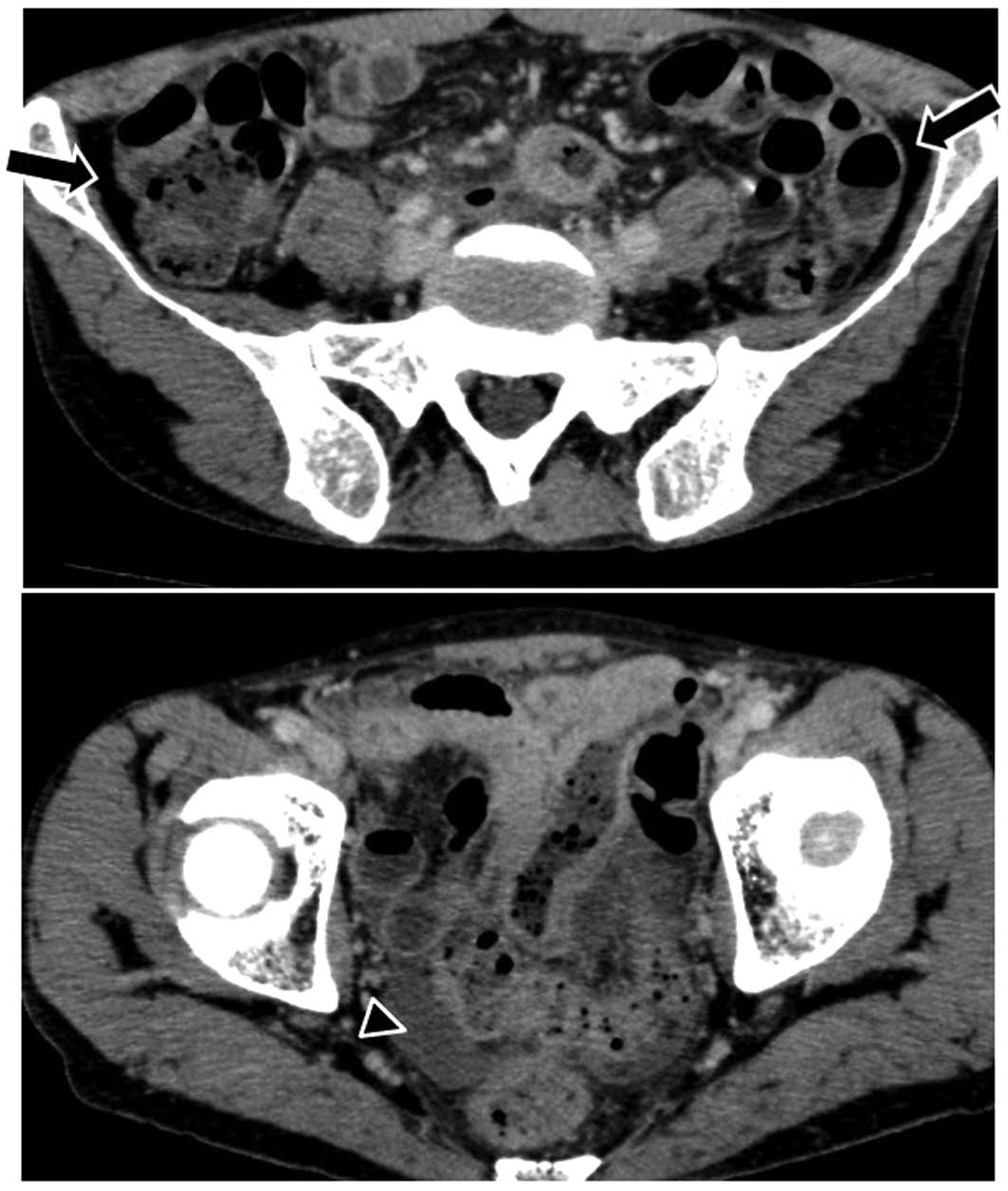
the patient experienced sudden abdominal pain. An abdominal CT scan
revealed free air and ascitic fluid in the pouch of Douglas
(Fig. 5). Although the site of
perforation was not identified, the patient was diagnosed with
acute peritonitis with gastrointestinal perforation and underwent
emergency surgery. During the surgical procedure, a large amount of
turbid ascites was found. A perforation was discovered inside the
jejunum, ~33.0 cm from Treitz's ligament. An intestinal resection
of ~10 cm was performed. The intraperitoneal cavity was washed with
8,000 ml saline solution and a functional end-to-end anastomosis
was performed. After the operation the patient's condition improved
gradually. On pathological examination, the resected tumor was
diagnosed as metastatic pleomorphic carcinoma of the lung, which
was negative for G-CSF immunostaining. This result may be
reflective of a possibly heterogeneous tumor population, in which
metastatic tumor cells did not produce G-CSF. One month after the
surgery, WBC and CRP increased to >105,100/µl and 39.06 mg/dl,
respectively, and the patient succumbed to the disease 8 months
after the initiation of the chemotherapy.
Discussion
Pleomorphic carcinomas are infrequent, comprising
0.1–0.3% of all lung tumors (1–3). Several
studies have reported that pleomorphic carcinomas are associated
with a more advanced stage at presentation and a poorer prognosis
(1,5,8,9). Recent data indicate that
pemetrexed-containing chemotherapy achieves a good response with
prolonged survival in advanced conventional NSCLC (10). By contrast, there is a lack of
knowledge regarding the role of chemotherapy in the treatment of
locally advanced, metastatic, and recurrent pleomorphic carcinoma
of the lung.
Bae et al reported that the current strategy
of palliative chemotherapy may be largely ineffective and
palliative chemotherapy itself is not justifiable (8). The median overall survival (OS) of their
11 patients who did not receive palliative chemotherapy for
advanced pulmonary pleomorphic carcinoma was only 2 months,
compared with 8 months for the patients who received palliative
chemotherapy (8). Hong et al
reported the role of palliative chemotherapy for 12 patients with
advanced pulmonary pleomorphic carcinoma (11) and demonstrated that the median OS from
the day of initiation of first-line chemotherapy was 8 months
(11). Of the 12 patients, 3 survived
for 1 year and were treated with TKIs as third-line therapy,
although the epidermal growth factor receptor (EGFR) mutation
status was not described in these patients. Interestingly, 1 of the
3 patients was treated with pemetrexed as second-line chemotherapy
(11). In the present study, we
demonstrated a sustained response for 30 months in 1 patient and
stable disease for 6 months without deterioration of the general
condition in another patient treated with pemetrexed-containing
chemotherapy. As both patients had wild-type EGFR mutations, they
received no TKI therapy. It is well known that the adverse events
of pemetrexed are milder compared with those observed with other
cytotoxic drugs, such as taxanes and vinorelbine (12). Despite the prolonged administration of
pemetrexed and bevacizumab in case 1, there were no severe adverse
events >grade 3, suggesting that cumulative dosing of these
drugs was not associated with severe adverse events.
In case 2, the patient had a G-CSF-producing
pleomorphic carcinoma of the lung. To the best of our knowledge,
there are no reported treatment results for G-CSF-producing
pleomorphic carcinoma of the lung. It is well known that
G-CSG-producing tumors tend to have poor prognosis (13,14). The
patient was treated with 5 courses of pemetrexed- and
bevacizumab-containing chemotherapy. Although the primary tumor
exhibited no shrinkage with chemotherapy, prevention of rapid
progression and stable disease for 6 months without deterioration
of the general condition of the patient was achieved. However,
rapid growth of the primary tumor was observed after stable
disease, with development of metastases to multiple sites,
including the small intestine. We hypothesized that the rapid
progression of the disease, including the small intestinal
metastasis in our patient, was associated with malignant
transformation of the tumor (15).
The outcome of the chemotherapy in this case was not satisfactory,
although chemotherapy was somewhat effective, without any severe
adverse events.
Our cases demonstrated the potential utility of
pemetrexed- and bevacizumab-containing chemotherapy. Our results
also suggest that pemetrexed-containing chemotherapy may be key to
the treatment of pleomorphic carcinoma of the lung.
References
|
1
|
Chang YL, Lee YC, Shih JY and Wu CT:
Pulmonary pleomorphic (spindle) cell carcinoma: Peculiar
clinicopathologic manifestations different from ordinary non-small
cell carcinoma. Lung Cancer. 34:91–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito K, Oizumi S, Fukumoto S, Harada M,
Ishida T, Fujita Y, Harada T, Kojima T, Yokouchi H and Nishimura M:
Hokkaido Lung Cancer Clinical Study Group: Clinical characteristics
of pleomorphic carcinoma of the lung. Lung Cancer. 68:204–210.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pelosi G, Sonzogni A, De Pas T, Galetta D,
Veronesi G, Spaggiari L, Manzotti M, Fumagalli C, Bresaola E, Nappi
O, et al: Review article: Pulmonary sarcomatoid carcinomas: A
practical overview. Int J Surg Pathol. 18:103–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mochizuki T, Ishii G, Nagai K, Yoshida J,
Nishimura M, Mizuno T, Yokose T, Suzuki K and Ochiai A: Pleomorphic
carcinoma of the lung: Clinicopathological characteristics of 70
cases. Am J Surg Pathol. 32:1727–1735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fishback NF, Travis WD, Moran CA, Guinee
DG Jr, McCarthy WF and Koss MN: Pleomorphic (spindle/giant cell)
carcinoma of the lung. A clinicopathologic correlation of 78 cases.
Cancer. 73:2936–2945. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi G, Cavazza A, Sturn N, Migaldi M,
Facciolongo N, Longo L, Maiorana A and Brambilla E: Pulmonary
carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements:
A clinicopathlogic and immunohistochemical study of 75 cases. Am J
Surg Pathol. 27:311–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto S, Hamatake D, Ueno T, Higuchi T,
Hiratsuka M, Shiraishi T, Iwasaki A and Shirakusa T:
Clinicopathological investigation of pulmonary pleomorphic
carcinoma. Eur J Cardiothorac Surg. 32:873–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bae HM, Min HS, Lee SH, Kim DW, Chung DH,
Lee JS, Kim YW and Heo DS: Palliative chemotherapy for pulmonary
pleomorphic carcinoma. Lung Cancer. 58:112–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raveglia F, Mezzetti M, Panigalli T, Furia
S, Giuliani L, Conforti S and Meda S: Personal experience in
surgical management of pulmonary pleomorphic carcinoma. Ann Thorac
Surg. 78:1742–1747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuld AD, Dragnev KH and Rigas JR:
Pemetrexed in advanced non-small-cell lung cancer. Expert Opin
Pharmacother. 11:1387–1402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong JY, Choi MK, Uhm JE, Park MJ, Lee J,
Park YH, Ahn JS, Park K, Han JH and Ahn MJ: The role of palliative
chemotherapy for advanced pulmonary pleomorphic carcinoma. Med
Oncol. 26:287–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Wen Q, Liu H, Ao R, Wu X, Guo L,
Wang W, He C and Wang J: Pemetrexed versus vinorelbine treatment of
advanced non-squamous non-small cell lung cancer in elderly
patients. Mol Clin Oncol. 1:553–557. 2013.PubMed/NCBI
|
|
13
|
Shijubo N, Inoue Y, Hirasawa M, Igarashi
T, Mori M, Matsuura A, Uede T and Suzuki A: Granulocyte
colony-stimulating factor-producing large cell undifferentiated
carcinoma of the lung. Intern Med. 31:277–280. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto M, Manabe S, Moriyama Y, Ishii H,
Tanaka S, Takahashi R, Tomaru K, Kobayashi N, Kudo M, Sasaki M, et
al: Long-term remission achieved via combined chemotherapy and
radiotherapy in a non-resectable granulocyte colony-stimulating
factor producing pleomorphic carcinoma of the lung. Intern Med.
52:2259–2263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao L: Recent advances in the molecular
diagnosis of lung cancer. Oncogene. 21:6960–6969. 2002. View Article : Google Scholar : PubMed/NCBI
|