Introduction
Malignant fibrous histiocytoma (MFH) was first
described by Ozzelo et al (1)
and by O'Brien and Stout (2). MFH
lesions are composed of different proportions of differentiated
fibroblasts and histiocytes arranged in a storiform pattern
(2). MFH is the most common soft
tissue sarcoma in adults and is generally considered a high-grade
sarcoma with poor prognosis (3,4). MFH may
occur at a wide range of ages, ranging mainly from 20 to 70 years.
MFHs most commonly occur in the lower extremities and the
retroperitoneum, whereas they are relatively uncommon in the head
and neck, accounting for 3–10% of all MFHs (5). Thus far, there have been only a limited
number of case reports on MFHs in the head and neck, and only a few
studies have included a description of the radiological findings
(6–9).
Due to its rare occurrence, there are few reports focusing on the
computed tomography (CT) or magnetic resonance imaging (MRI)
findings of MFH of the head and neck. Park et al (10) first reported the imaging
characteristics of 13 patients with histologically diagnosed MFH of
the head and neck, although the availability of information on the
imaging characteristics of MFH remains limited. The purpose of our
study was to present the CT and MR imaging characteristics of 15
cases of MFH arising in the head and neck.
Patients and methods
Patients
This retrospective study was approved by the
Institutional Review Board of Sun Yat-Sen University (Guangzhou,
China). Data were retrospectively collected from the medical
records of patients with MFH histologically diagnosed at the Sun
Yat-sen University Cancer Center between 2006 and 2013. The study
included a total of 15 patients (9 men and 6 women), ranging in age
from 20 to 77 years, with a mean age of 50 years. Among these
patients, 4 developed MFH following radiotherapy for nasopharyngeal
carcinoma or chondrosarcoma; the remaining patients were diagnosed
with primary MFH. In 6 cases, the tumor recurred between 5 months
and 2 years after surgery. All the MRI and CT images were reviewed
and analyzed.
Imaging techniques
MRI scans were performed in 4 patients and CT scans
in 11 patients. MR imaging was performed using a 1.5T system (Signa
Excite II 1.5; GE Medical Systems, Waukesha, WI, USA) or a 3.0T
system (Trio Tim; Siemens Medical Solutions, Erlangen, Germany)
with a head and neck combined coil. Non-contrast-enhanced
T1-weighted images (TR=500–600 msec, TE=10–20 ms) in the axial,
coronal and sagittal planes, and non-contrast-enhanced T2-weighted
images (TR=4,000–6,000 msec, TE=95–110 msec) in the axial plane
were obtained. Following intravenous injection of gadopentetate
dimeglumine at a dose of 0.1 mmol/kg body weight, T1-weighted
(TR=320–350 msec, TE=10–20 msec) fat-suppressed axial, coronal and
sagittal sequences were obtained sequentially, with a 5-mm section
thickness and 1-mm intersection gaps.
CT scans were obtained with a Brilliance TM16
(Philips Medical Systems, Best, The Netherlands) or a Toshiba
Aquillion TM64 (Toshiba Medical Systems, Otawara, Japan) helical CT
system. The main imaging parameters were as follows: 120 kV,
250–300 mA; section thickness, 5 mm; field of view, 25 cm; and
matrix, 512×512. An intravenous bolus dose of 90 ml non-ionic
iodinated contrast agent (iopromide; Ultravist, Schering, Berlin,
Germany) was administered at a rate of 3 ml/sec.
Two experienced radiologists reviewed all the CT and
MRI images and analyzed the imaging characteristics, including
tumor location, size, margin, internal architecture, density or
signal intensity and enhancement pattern.
Results
Tumor characteristics
Of the 15 MFHs in the head and neck in our series, 5
cases were located in the maxillary sinus, 1 in the ethmoid sinus,
2 in the infratemporal fossa, 4 in the neck, 1 in the left
mandible, 1 in the gingiva and 1 in the epiglottis.
There were 7 lesions with histological subtypes that
were classifiable, including 4 inflammatory, 2 storiform-pleomophic
and 1 undifferentiated. Of the 4 inflammatory types, 1 case had
undergone an MRI scan, and 3 cases had undergone CT scans. One case
of the storiform-pleomophic type had undergone CT scan at first
presentation and a subsequent MRI scan when the tumor recurred.
Imaging findings
The CT/MRI imaging findings of the 15 MFHs are
presented in the Table I. All the
lesions appeared as ill-defined, ranging in size from 2.1 to 5.1 cm
in the largest diameter. Six sinonasal lesions, 2 infratemporal
fossa lesions and 1 mandibular lesion were accompanied by bone
fracture. On unenhanced CT images, the lesions (n=11) were
isoattenuated relative to the adjacent muscle; 2/11 lesions were
homogeneous and 9/11 lesions were heterogeneous, with areas of
hypoattenuation. Calcifications were observed in 1 case that
received irradiation treatment for primary chondrosarcoma.
Following injection of contrast agent, 5 lesions exhibited
heterogeneous mild-moderate enhancement, 5 exhibited heterogeneous
marked enhancement, and 1 lesion exhibited ring-like marked
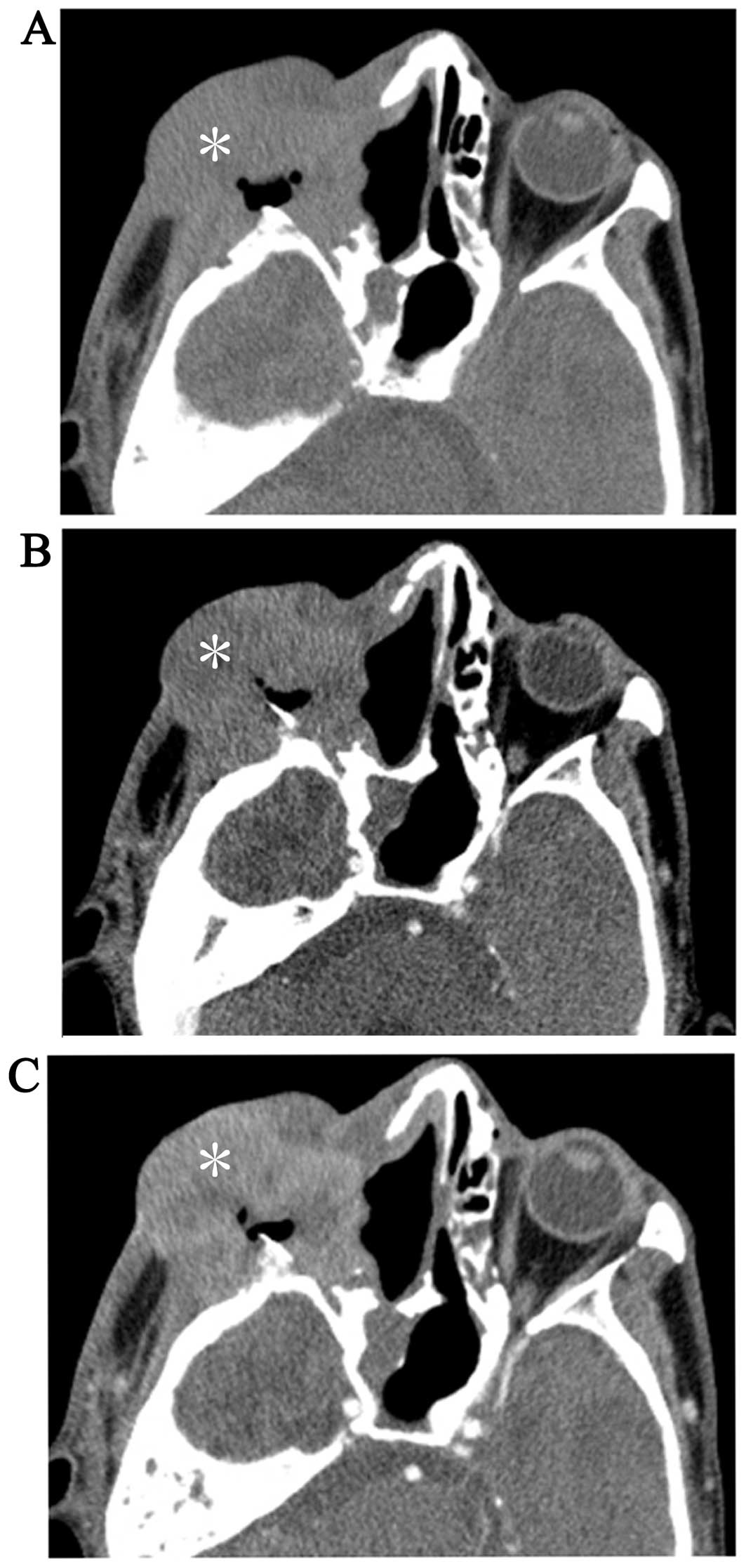
enhancement. There were only 2 cases in which dual-phase enhanced
CT scans were performed: One was an inflammatory MFH and exhibited
markedly prolonged enhancement (Fig.
1); the subtype of the other could not be classified and
exhibited mildly prolonged enhancement (Fig. 2).
 | Table I.CT/MRI imaging findings of 15 cases
with MFH of the head and neck. |
Table I.
CT/MRI imaging findings of 15 cases
with MFH of the head and neck.
| Age (yrs)/gender | Imaging modality | Location | Margin | Density in CT | T1WI | T2WI | Enhancement | Bone destruction | Histological
subtype |
|---|
| 74/M | MRI | Left mandible,
infratemporal fossa | Ill-defined | – | Heterogeneous
isointense | Heterogeneous
hyperintense, necrosis | Heterogeneous,
marked | Yes | – |
| 25/M | CT | Left maxillary sinus,
infratemporal fossa | Ill-defined | Heterogeneous
isodense | – | – | Heterogeneous,
mild | Yes | – |
| 20/M | CT | Left ethmoid sinus,
endocanthion | Ill-defined | Heterogeneous
isodense | – | – | Heterogeneous,
mild | Yes | – |
| 47/M | CT | Epiglottis | Ill-defined | Homogeneous
isodense | – | – | Heterogeneous,
mild-moderate | No | – |
| 73/M | CT | Right neck | Ill-defined | Heterogeneous
isodense | – | – | Heterogeneous,
moderate | No | – |
| 77/M | CT | Right neck, right
parotid gland | Ill-defined | Heterogeneous
isodense | – | – | Heterogeneous,
marked | No | Inflammatory |
| 41/M | CT | Right maxillary
sinus, right eyeball | ill-defined | Heterogeneous
isodense | – | – | Heterogeneous,
moderate-marked | Yes | – |
| 44/F | CT | Gingiva | Ill-defined | Homogeneous
isodense | – | – | Heterogeneous,
mild | No |
Storiform-pleomophic |
| 45/F | CT | Right maxillary
sinus | Ill-defined | Heterogeneous
isodense, calcificaiton | – | – | Heterogeneous,
mild | Yes | Inflammatory |
| 48/M | MRI | Left infratemporal
fossa | Ill-defined | – | Heterogeneous
isointense | Heterogeneous obvious
hyperintense, necrosis | Ring-like | Yes | – |
| 41/F | CT/MRI | Left of neck | Ill-defined | Heterogeneous
isodense | Homogeneous
isointense | Homogeneous
isointense | Heterogeneous,
mild | No |
Storiform-pleomophic |
| 45/F | CT | Left infratemporal
fossa | Ill-defined | Heterogeneous
isodense, lightly hypodense | – | – | Ring-like | Yes | Undifferentiated |
| 61/F | CT | Right maxillary
sinus | Ill-defined | Homogeneous
isodense |
|
| Heterogeneous,
marked | Yes | Inflammatory |
| 55/F | CT | Left maxillary
sinus | Ill-defined | Heterogeneous
isodense |
|
| Heterogeneous,
mild | Yes | – |
On T1-weighted images, the lesions (n=4) were
isointense, with small foci of hyperintensity in 2 cases and
heterogeneous isointensity in 2 cases. On T2-weighted images, all
the lesions exhibited heterogeneous hyperintensity. One case
exhibited small foci of short-T2 signals that were histologically
identified as calcifications (Fig.
3). On post-contrast images, the lesions exhibited
heterogeneous moderate-marked enhancement in 3 cases and ring-like
marked enhancement in 1 case (Fig.
4). The non-enhancement areas represented necrosis in 2
ring-like enhancement lesions.
Discussion
MFH is a potentially devastating sarcoma that may
occur at any age, although it is particularly prevalent in the
fifth to seventh decades of life, with a male predominance. MFH
frequently arises in the lower extremities, followed by the upper
extremities and retroperitoneum (11). The incidence of MFHs in the head and
neck is relatively low, accounting for only 3–10% of all MFHs
according to the literature (5). The
sinonasal tract has been reported to be the most common site of MFH
involvement in this region (12,13). In
our series, the most common sites were the maxillary sinus (5/15),
the neck (4/15) and the infratemporal fossa (2/15). MFH is reported
to be one of the most common soft tissue sarcomas in adults; the
majority of MFHs are high-grade lesions and frequently recur
locally. Surgery is considered the primary treatment for MFH in the
head and neck region, although the addition of chemotherapy and
radiotherapy appears to be helpful for certain patients (14,15).
Lymphatic metastases are also rare, ranging between 0 and 15%
(11). Only 1 case exhibited
lymphatic metastasis in our series. All the cases underwent
surgical excision; in 6 cases the tumor recurred, whereas the
remaining cases were lost to follow-up.
MFH is reported to be the most common
radiation-induced sarcoma (RIS) in the head and neck region
(16). However, there are no studies
demonstrating the association between specific radiation doses and
RIS. Some researchers (17) have
suggested that a total dose of ≥55 Gy may increase the risk of RIS.
Cai et al (18) described 59
cases of RIS in the head and neck, including 10 (16.9%) MFHs, which
ranked third in the series; the first and second most common types
of RIS were fibrosarcoma and osteosarcoma, respectively. There were
4 cases of post-irradiation MFH in our series; the remaining cases
were primary MFHs.
Clinically, patient symptoms often depend on the
location of the tumor and its proximity to adjacent structures.
Pain is the main symptom of MFH in the sinonasal tract due to the
limited size of the sinus cavity; the sinus walls are always
involved as the tumor grows. Patients may also present with nasal
obstruction and epistaxis. MFH in the neck usually presents as a
painless, enlarging mass.
MFH was first described in 1963, and the debate over
the origin of the tumor cells has persisted for several years. MFH
is currently considered to be synonymous with high-grade
undifferentiated pleomorphic sarcoma. MFH was previously subdivided
into five types: i) Storiform-pleomophic; ii) myxoid; iii)
inflammatory; iv) giant-cell; and v) angiomatoid. However,
according to the 2002 World Health Organization classification, the
subtypes of MFH have been revised. For example, myxoid MFH and
angiomatoid MFH are no longer considered to be subtypes of MFH,
whereas giant-cell MFH has been renamed undifferentiated
pleomorphic sarcoma with giant cells, with the diagnosis made in
the absence of differentiation. Inflammatory MFH was also renamed
undifferentiated pleomorphic sarcoma with prominent inflammation,
and this diagnosis is confirmed only if all the markers of
mesenchymal lineage are negative. Storiform-pleomorphic MFH remains
a category of fibrohistiocytic tumors (19,20) and is
the most common subtype in the head and neck region.
MFHs lack typical CT/MRI imaging characteristics due
to the variable degrees of histological differentiation. In
particular, the density/signal intensity and enhancement pattern on
CT/MRI are varied and exhibit no differences from other malignant
soft tissue tumors. The tumor typically presents as a round mass
with ill-defined margins and always invades the adjacent bones and
soft tissue. The density/signal intensity is typically
heterogeneous, although necrosis may be observed in the lesions,
appearing as lightly hypodense/marked hyperintense regions on
T2-weighted images without enhancement. Calcification or
ossification may also be observed in ~5–20% of these lesions
(10). In our series, necrosis was
detected in 5 of the 15 cases. Calcification was detected on CT
images in 1 case with prior osteosarcoma; the other case that
underwent MRI also exhibited calcification in the pathological
specimen, which appeared as a hypointense lesion on T2-weighted
images. The tumors exhibited different degrees of heterogeneous
enhancement on CT and MRI images. Park et al (10) analyzed 13 cases of MFH and reported
that the signal and enhancement patterns of MFH were non-specific,
with the exception of the myxoid type, which exhibited homogeneous
marked hyperintensity on T2-weighted images and homogeneous marked
enhancement. There were no myxoid-type MFH cases in our series;
however, there were 4 cases of inflammatory MFH that exhibited
marked enhancement, and 1 case exhibited prolonged enhancement in
the venous phase. This enhancement pattern has not been previously
reported and may suggest that inflammatory MFH is hypervascular.
However, not all inflammatory MFHs exhibit marked enhancement.
Karki et al (21) reported 3
cases of inflammatory MFH, although only 1 case exhibited marked
enhancement, whereas the remaining 2 cases exhibited mild
enhancement. These different enhancement patterns may be the result
of different tumor locations.
In conclusion, the imaging findings of MFH are
non-specific. However, inflammatory MFH of the head and neck may
exhibit marked and prolonged enhancement. The diagnosis of MFH
should be considered if the patient presents with a history of
radiotherapy.
References
|
1
|
Ozzello L, Stout AP and Murray MR:
Cultural characteristics of malignant histiocytomas and fibrous
xanthomas. Cancer. 16:331–344. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Brien JE and Stout AP: Malignant fibrous
xanthomas. Cancer. 17:1445–1455. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wanebo HJ, Koness RJ, MacFarlane JK,
Eilber FR, Byers RM, Elias EG and Spiro RH: Head and neck sarcoma:
Report of the Head and Neck Sarcoma Registry. Society of Head and
Neck Surgeons Committee on Research. Head Neck. 14:1–7. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potter BO and Sturgis EM: Sarcomas of the
head and neck. Surg Oncol Clin N Am. 12:379–417. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sturgis EM and Potter BO: Sarcomas of the
head and neck region. Curr Opin Oncol. 15:239–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagi Y, Murakami J, Hisatomi M, Katase
N, Nagatsuka H and Asaumi J: A case of malignant fibrous
histiocytoma of the maxillary sinus. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 109:e99–e104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senel FC, Bektas D, Caylan R, Onder E and
Gunhan O: Malignant fibrous histiocytoma of the mandible.
Dentomaxillofac Radiol. 35:125–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagano H, Deguchi K and Kurono Y:
Malignant fibrous histiocytoma of the bucca: A case report. Auris
Nasus Larynx. 35:165–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satomi T, Watanabe M, Kaneko T,
Matsubayashi J, Nagao T and Chiba H: Radiation-induced malignant
fibrous histiocytoma of the maxilla. Odontology. 99:203–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SW, Kim HJ, Lee JH and Ko YH:
Malignant fibrous histiocytoma of the head and neck: CT and MR
imaging findings. AJNR Am J Neuroradiol. 30:71–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss SW and Enzinger FM: Malignant
fibrous histiocytoma: An analysis of 200 cases. Cancer.
41:2250–2266. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabesan T, Xuexi W, Yongfa Q, Pingzhang T
and Ilankovan V: Malignant fibrous histiocytoma: Outcome of tumours
in the head and neck compared with those in the trunk and
extremities. Br J Oral Maxillofac Surg. 44:209–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodrigo JP, Fernández JA, Suárez C, Gómez
J, Llorente JL and Herrero A: Malignant fibrous histiocytoma of the
nasal cavity and paranasal sinuses. Am J Rhinol. 14:427–431. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clark DW, Moore BA, Patel SR, Guadagnolo
BA, Roberts DB and Sturgis EM: Malignant fibrous histiocytoma of
the head and neck region. Head Neck. 33:303–308. 2011.PubMed/NCBI
|
|
15
|
Hardison SA, Davis PL III and Browne JD:
Malignant fibrous histiocytoma of the head and neck: A case series.
Am J Otolaryngol. 34:10–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cakir O, Topal U, Bayram AS and Tolunay S:
Sarcomas: Rare primary malignant tumors of the thorax. Diagn Interv
Radiol. 11:23–27. 2005.PubMed/NCBI
|
|
17
|
Patel SG, See AC, Williamson PA, Archer DJ
and Evans PH: Radiation induced sarcoma of the head and neck. Head
Neck. 21:346–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai PQ, Wu YP, Li L, Zhang R, Xie CM, Wu
PH and Xu JH: CT and MRI of radiation-induced sarcomas of the head
and neck following radiotherapy for nasopharyngeal carcinoma. Clin
Radiol:. 68:683–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matushansky I, Charytonowicz E, Mills J,
Siddiqi S, Hricik T and Cordon-Cardo C: MFH classification:
Differentiating undifferentiated pleomorphic sarcoma in the 21st
century. Expert Rev Anticancer Ther. 9:1135–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Agha OM and Igbokwe AA: Malignant
fibrous histiocytoma: Between the past and the present. Arch Pathol
Lab Med. 132:1030–1035. 2008.PubMed/NCBI
|
|
21
|
Karki B, Xu YK, Wu YK and Zhang WW:
Primary malignant fibrous histiocytoma of the abdominal cavity: CT
findings and pathological correlation. World J Radiol. 4:151–158.
2012. View Article : Google Scholar : PubMed/NCBI
|