Introduction
Leukocytoclastic vasculitis (LCV) has been
associated with several disease processes, such as infections,
drugs, allergies, rheumatological and neoplastic diseases (1). In neoplastic processes, LCV may present
as a paraneoplastic phenomenon occurring before, synchronously
with, or after the diagnosis of malignancy (2). Compared to hematological malignancies,
solid tumors are significantly less likely to be associated with
LCV (1). The first case of LCV in a
patient with a solid tumor was reported in 1968 (3). Since then, more patients suffering from
solid tumors and vasculitis have been documented in the literature
(4,5),
including patients with lung, colon, gastric and renal cancer. In
these previously reported cases, the patients only developed one
type of malignancy and the occurrence of multiple malignancies in
patients with LCV has not been reported. We herein describe the
case of a patient who developed LCV and was treated with
glucocorticoids for 11 years. The patient then developed three
primary tumors, including small-cell lung carcinoma (SCLC), gastric
adenocarcinoma and colonic adenocarcinoma. To the best of our
knowledge, this is the first case report of multiple malignancies
developing in a patient with a history of LCV in the English
medical literature.
Case report
The patient was a 54-year-old man, initially
presenting with rash, purpura, petechiae, bruising and desquamation
on the right low limb followed by ulceration in May, 2001 at the
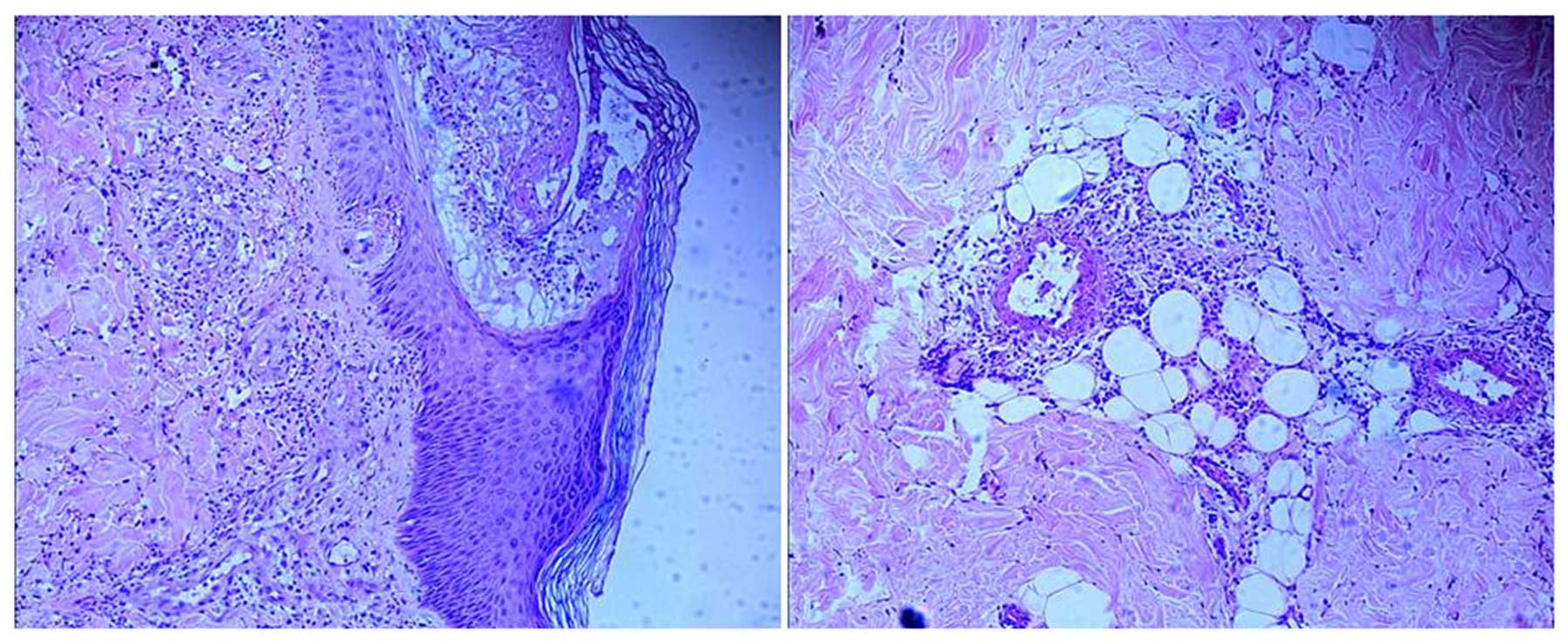
age of 43 years. Skin biopsy confirmed the diagnosis of LCV
(Fig. 1). The patient was treated
with glucocorticoids and initially responded to the steroid therapy
very well, achieving complete response. However, the disease later
relapsed, and the patient developed dry gangrene of the right foot.
Shock therapy with a maximum dose of 500 mg methylprednisolone for
5 days was administered repeatedly. After the disease was
controlled, the patient was maintained on methylprednisolone at
doses of 80, 60, 40, 20 and 4 mg, adjusted according to the disease
status over the following 11 years.
In February, 2012, approximately 11 years after the
diagnosis of LCV, the patient presented to our hospital complaining
of nausea and paroxysmal dull pain in the back and upper abdomen
for 3 weeks. The LCV had been stabilized on 4 mg methylprednisolone
twice a day. The patient was not a smoker and had no family history
of cancer. On physical examination, the patient had a moon facies.
No hepatosplenomegaly or lymph node enlargement were observed.
There was no jaundice, rash or ulceration. The lower part of the
right leg exhibited purple discoloration and desquamation, with
marked deformity of the toes, possibly due to the dry gangrene the
patient developed due to the deterioration of his LCV (Fig. 2).
The complete blood count revealed a white blood cell
count of 10.9×109/l, with an absolute neutrophil count
of 8.76×109/l. The percentage of neutrophils and
lymphocytes was 80.4 and 8.6%, respectively. The hemoglobin level
and platelet count were within normal limits. Routine urine tests
showed no positive findings, while the routine stool test was
positive for occult blood. Blood chemistry tests revealed an
alkaline kinase proteinase level of 341 U/l (normal, 42–141 U/l),
γ-GT 263 U/l (normal, 7–32 U/l) and lactate dehydrogenase 624.50
U/l (normal, 22–29 U/l); others were within the normal range. Tumor
marker tests revealed an α-fetoprotein level of 3.0 ng/ml (normal,
<10.9 ng/ml), carcinoembryonic antigen 3.63 ng/ml (normal, <5
ng/ml), ferritin >1,500 ng/ml (normal, 11–306.8 ng/ml),
carbohydrate antigen (CA)125 >1,000 U/ml (normal, <35 U/ml),
CA153, 78.91 U/ml (normal, <31.3 U/ml) and CA199, 112.28 U/ml
(normal, <37 U/ml). The results of the cellular immunity tests
were as follows: CD3, 37% (normal, 50–84%); CD19, 4% (normal,
5–18%), CD4, 20% (normal, 27–51%), CD8, 50% (normal, 15–44%); and
CD4/CD8 ratio, 0.4 (normal, 0.71–2.78).
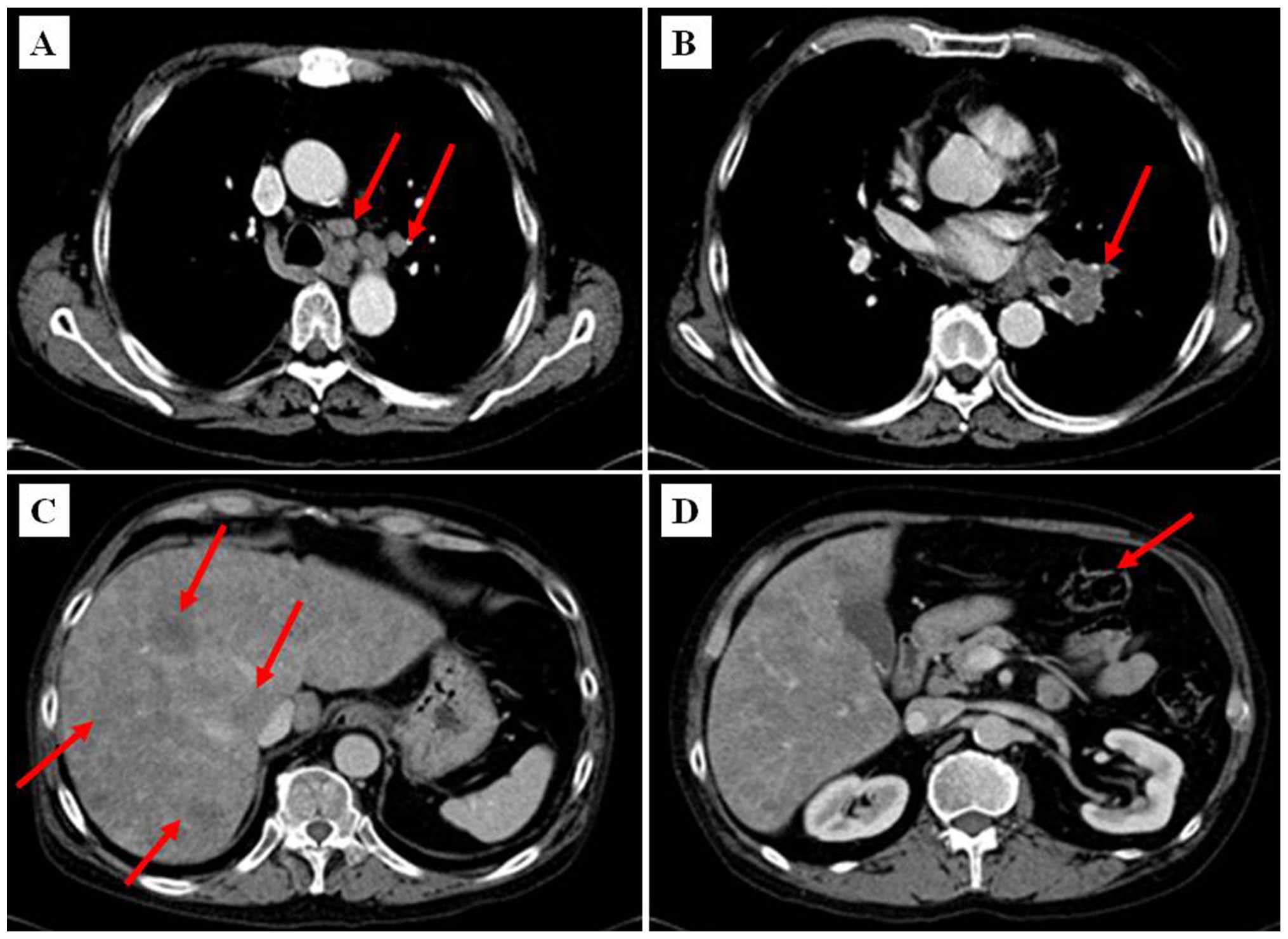
A CT scan of the chest and abdomen was performed and
revealed bulky disease at the left lung hilus sized ≤4.2–5.6 cm,
enlarged mediastinal lymph nodes sized ≤2–3 cm (Fig. 3A–B) and diffuse liver metastases
(Fig. 3C). In addition, minor
incrassation of the gastric wall in the antrum of stomach was
present (Fig. 3D).
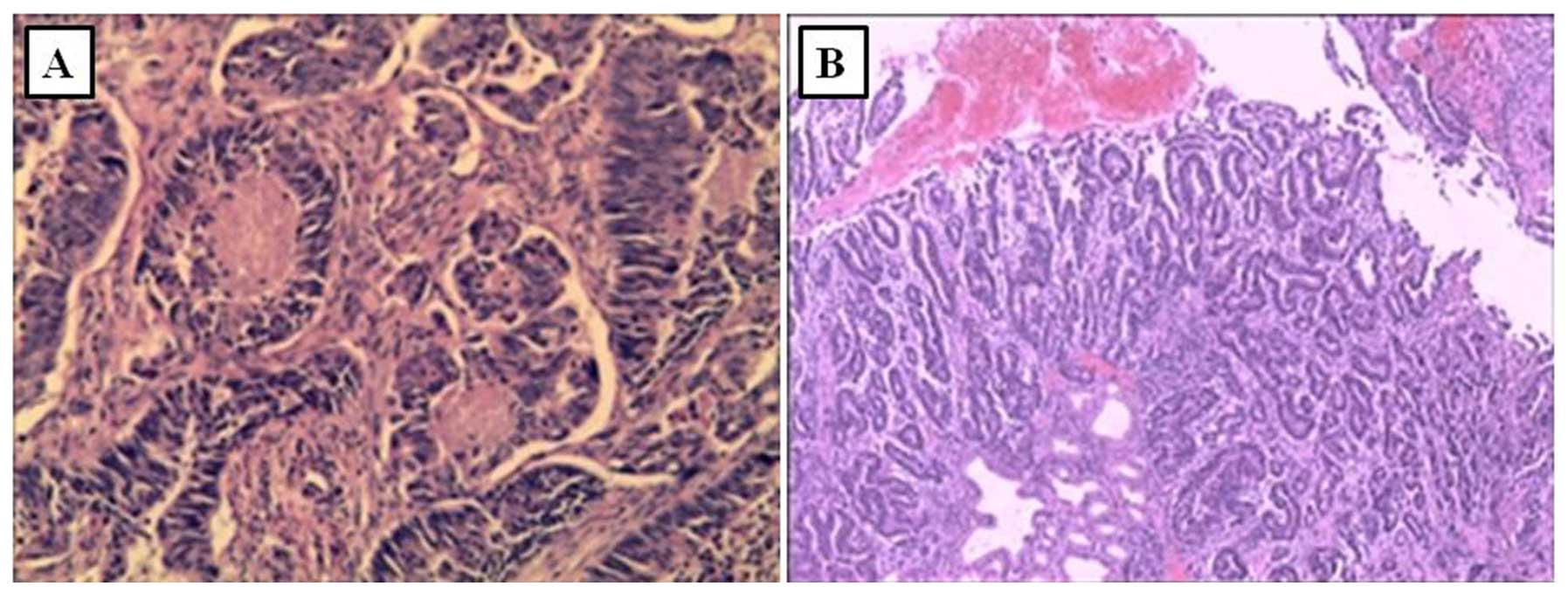
Colonoscopy and esophogastroduodenoscopy with
concurrent biopsies were performed. Colonoscopy revealed an
ulcerated lesion at the sigmoid region, measuring ~1×1.5 cm. Biopsy
of the lesion (Fig. 4A) showed
ulcerated type tubular adenocarcinoma. Esophogastroduodenoscopy
revealed antral ulceration; the examination for Helicobacter
pylori (H. pylori)was negative and the biopsy showed
gastric adenocarcinoma, moderately differentiated (Fig. 4B). To confirm the pathology of the
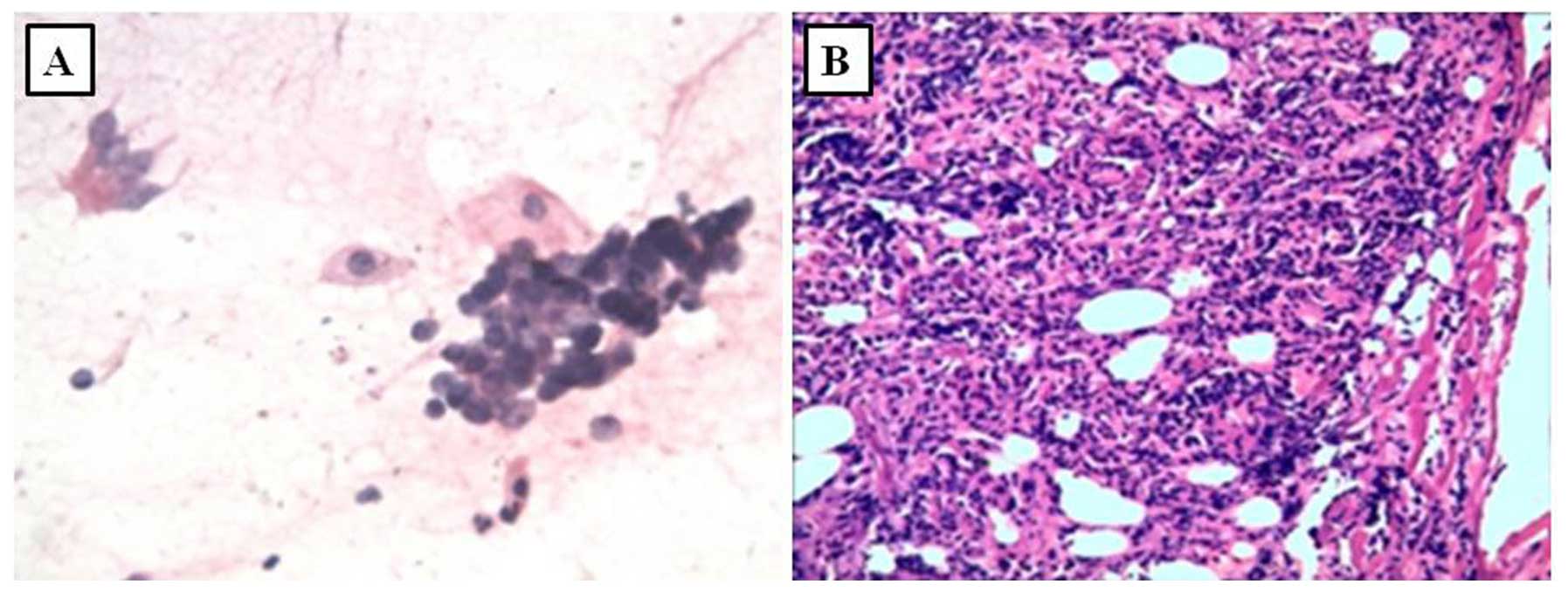
hilar mass in the left lung, bronchoscopy was performed and
revealed left hilar enlargement. A bronchial brushing smear
revealed clusters of malignant cells (Fig. 5A). SCLC was suspected and the
diagnosis was confirmed by biopsy (Fig.
5B).
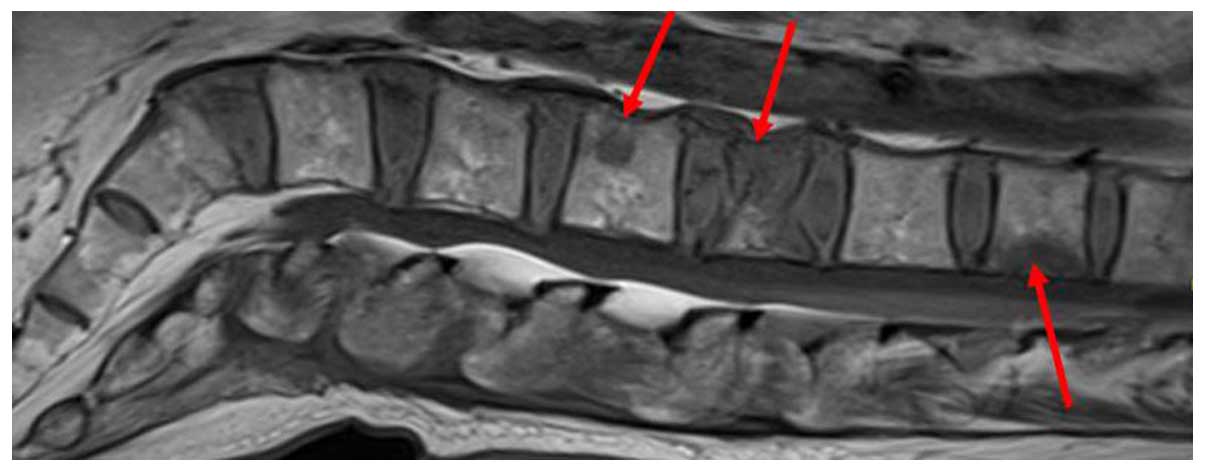
Due to back pain, vertebral magnetic resonance
imaging was performed, which revealed multiple metastases to the
spine, from the twelfth thoracic to the second sacral vertebra. A
vertebral compression fracture of the second lumbar vertebra was
also identified (Fig. 6).
In summary, the patient was diagnosed with three
separate maligancies: Gastric adenocarcinoma, colonic
adenocarcinoma and SCLC, with stage IV metastatic disease. The
patient received palliative chemotherapy, which included paclitaxel
120 mg (days 1 and 8), etoposide 100 mg (days 1–5) and cisplatin 20
mg (days 1–5). During the course of chemotherapy, grade 4 bone
marrow suppression ensued, complicated by pulmonary pseudomonas
aeruginosa infection. The patient was started on antibiotics
and chemotherapy was terminated. However, his condition
deteriorated rapidly, with pronounced abdominal distention,
continuously declining urine volume, liver enlargement and ascites.
During this period there was no progression of LCV and no new rash,
purpura, petechia or ulceration were identified. The patient
succumbed 3 months after the diagnosis of the cancers.
Discussion
LCV is a neutrophilic inflammation of the blood
vessels. As a paraneoplastic syndrome, it may occur prior to the
diagnosis of the primary tumor. The exact association between LCV
and malignancy has not been fully elucidated. Various mechanisms
have been proposed for tumor-associated LCV, including
antigen-antibody complexes that form in response to tumor antigens
and are deposited in vessel walls, resulting in inflammation
(6). Malignant neoplasms may also
increase blood viscosity, causing potential endothelial damage and
increasing contact time for immune complex deposition (7). LCV, when presenting as a paraneoplastic
syndrome, is usually refrractory to corticosteroid and
immunosuppressant treatment, whereas its symptoms improve with
effective treatment of the underlying malignancy. Moreover,
recurrence of LCV often occurs with the progression or metastasis
of the malignancy (8). In our case,
LCV occurred 11 years prior to the development of the malignancies.
The patient responded to methylprednisolone therapy and there was
no deterioration of LCV when the malignancies were diagnosed. Thus,
we hypothesize that LCV was not a paranoeplastic syndrome, but
rather an independent condition in our patient. However, it is
likely that the immunosuppression triggered by the long-term
treatment of LCV with glucocorticoids contributed to the
development of multiple malignancies.
It is well known that glucocorticoid administration
inhibits the immune system, including induction of apoptosis in T
lymphocytes (9) and inhibition of
natural killer cell activity (10).
These have been associated with increased susceptibility to tumor
development (11). Furthermore,
glucocorticoids appear to contribute to the shift of T helper cells
from the Th1 to the Th2 phenotype (9), which facilitates cancer escape from host
immune surveillance (12). It was
previously demonstrated that patients with immunological disorders
treated with glucocorticoids displayed a high incidence of
secondary cancers (13). In our case,
the patient had a history of LCV and received long-term
glucocorticoid treatment, with the maximum dose reaching 500 mg/day
of methylprednisolone. The disruption of the immune system by
steroid treatment is likely the cause of cancer development. Other
cancer risk factors were absent in this patient, as he was not a
smoker and there was no H. pylori infection identified in
the gastric biopsy.
To the best of our knowledge, this is the first
reported case of multiple malignancies developing after long-term
steroid treatment in a patient with LCV. LCV in this case was not a
paraneoplastic syndrome, and the development of the malignancies is
likely associated with immune dysregulation by the steroid
treatment. This case report suggests that clinicians should be
aware of the possible association of long-term use of
glucocorticoids with the development of secondary malignancies.
Acknowledgements
The present study was supported by grants from the
Natural Science Youth Foundation of Jiangsu Province (BK20141034)
and the Project of Administration of Traditional Chinese Medicine
Research of Jiangsu Province (LZ13051), the ‘Top Talented Personnel
in Six Profession’ grant in Jiangsu Province (2011-WS-049) and a
grant from the Jiangsu Province Hospital of Traditional Chinese
Medicine (2013, Y1008).
References
|
1
|
Langford CA: Vasculitis. J Allergy Clin
Immunol. 125(2 Suppl 2): S216–S225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fain O, Hamidou M, Cacoub P, Godeau B,
Wechsler B, Pariès J, Stirnemann J, Morin AS, Gatfosse M, Hanslik
T, et al: Vasculitides associated with malignancies: Analysis of
sixty patients. Arthritis Rheum. 57:1473–1480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torvik A and Berntzen AE: Necrotizing
vasculitis without visceral involvement: Postmortem examination of
three cases with affection of skeletal muscles and peripheral
nerves. Acta Med Scand. 184:69–77. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Podjasek JO, Wetter DA, Pittelkow MR and
Wada DA: Cutaneous small-vessel vasculitis associated with solid
organ malignancies: The Mayo Clinic experience, 1996 to 2009. J Am
Acad Dermatol. 66:e55–e65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kathula SK, Thomas DE, Anstadt MP and Khan
AU: Paraneoplastic cutaneous leukocytoclastic vasculitis and iron
deficiency anemia as the presenting features of squamous cell lung
carcinoma. J Clin Oncol. 29:e83–e85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greer JM, Longley S, Edwards NL, Elfenbein
GJ and Panush RS: Vasculitis associated with malignancy: Experience
with 13 patients and literature review. Medicine (Baltimore).
67:220–230. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magro CM and Crowson AN: A clinical and
histologic study of 37cases of immunoglobulin A-associated
vasculitis. Am J Dermatopathol. 21:234–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Guerrero J, Gutiérrez-Ureña S,
Vidaller A, Reyes E, Iglesias A and Alarcón-Segovia D: Vasculitis
as a paraneoplastic syndrome: Report of 11 cases and review of the
literature. J Rheumatol. 17:1458–1462. 1990.PubMed/NCBI
|
|
9
|
Tuckermann JP, Kleiman A, McPherson KG and
Reichardt HM: Molecular mechanisms of glucocorticoids in the
control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab
Sci. 42:71–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oshimi K, Gonda N, Sumiya M and Kano S:
Effects of corticosteroids on natural killer activity in systemic
lupus erythematosus. Clin Exp Immunol. 40:83–88. 1980.PubMed/NCBI
|
|
11
|
Riley V: Psychoneuroendocrine influences
on immunecompetence and neoplasia. Science. 212:1100–1109. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asselin-Paturel C, Echchakir H, Carayol G,
Gay F, Opolon P, Grunenwald D, Chouaib S and Mami-Chouaib F:
Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression
in tumor, TIL and PBL of non-small cell lung cancer patients. Int J
Cancer. 77:7–12. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klumb EM, Araújo ML Jr, Jesus GR, Santos
DB, Oliveira AV, Albuquerque EM and Macedo JM: Is higher prevalence
of cervical intraepithelial neoplasia in women with lupus due to
immunosuppression? J Clin Rheumatol. 16:153–157. 2010. View Article : Google Scholar : PubMed/NCBI
|