Introduction
Ampullary carcinomas represent 0.5% of all
gastrointestinal malignancies. They are more common in men compared
with in women, and are normally diagnosed in patients between 60
and 80-years old (1).
The vast majority of lesions reveal a glandular
growth pattern. Among these, two predominant subtypes of
adenocarcinoma have been identified, which show either
pancreaticobiliary (15–20%) or intestinal differentiation (50–80%)
(2,3).
Subclassification is clinically relevant, since in addition to
advanced tumour stage and poor differentiation, the
pancreaticobiliary subtype has been identified as an adverse
prognostic factor (2,4–6). Tumours
with neuroendocrine differentiation, including neuroendocrine
tumours and neuroendocrine carcinomas (NECs) are only rarely
observed at this site (4,7,8).
Mixed adenoneuroendocrine carcinomas (MANECs) are
rare biphasic tumour types, which have only anecdotally been
reported in the ampullary region (7,9–12). The current report described a patient
with ampullary MANEC, presenting with widespread metastatic
dissemination. Both primary and metastatic tissues are assessed by
histology, which has not been previously well-described.
Case report
Clinical presentation
A 66-year-old female presented with painless
jaundice for 2 days, unspecific abdominal pain and weight loss of 5
kg within the last three months, and was admitted to the Department
of Surgery, General Hospital (Linz, Austria). Laboratory analysis
revealed pathological liver function tests, with predominant
cholestatic profile, including hyperbilirubinemia.
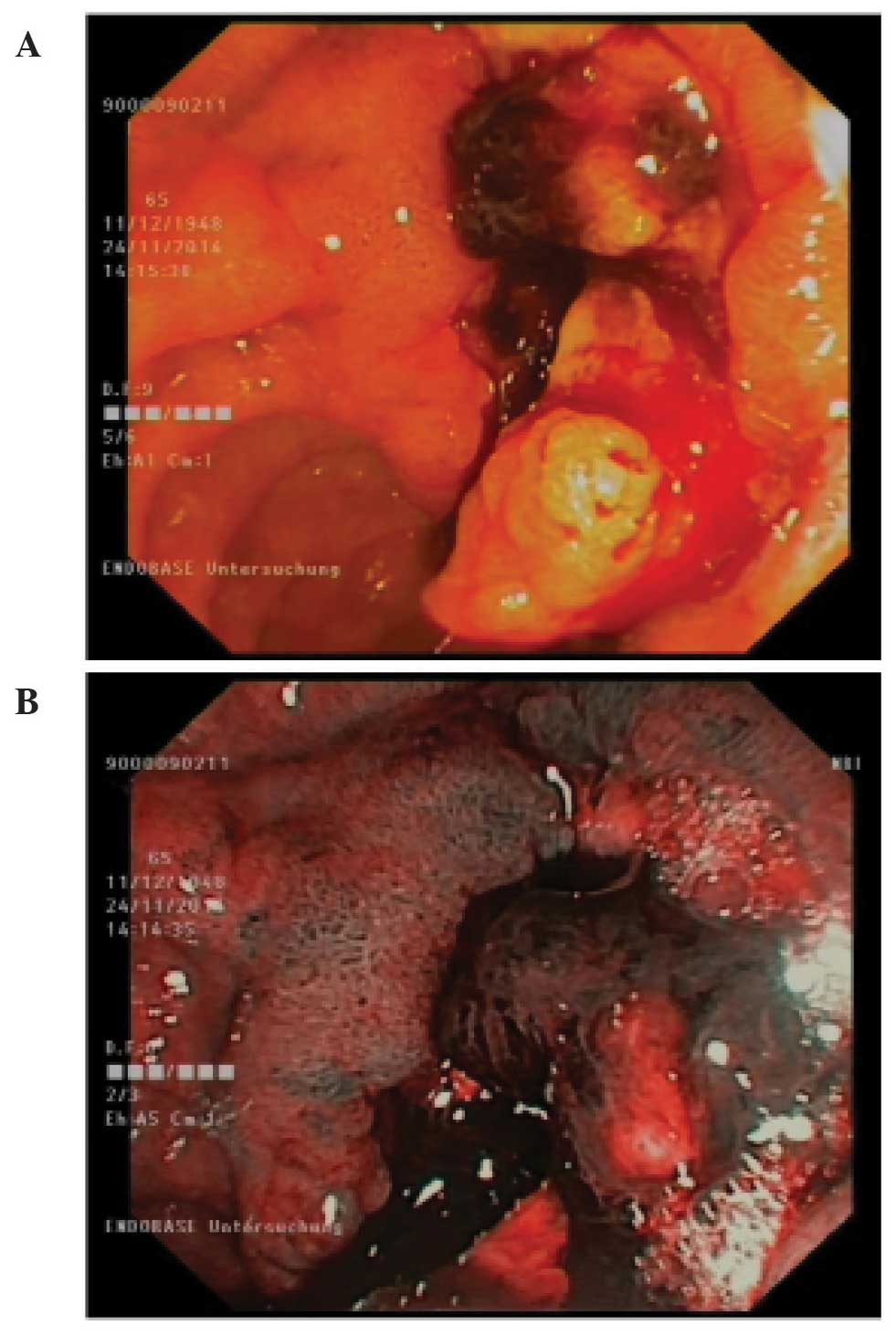
Upon endoscopy, an irregular tumour mass was
detected in the ampullary region, measuring ~4 cm in the largest
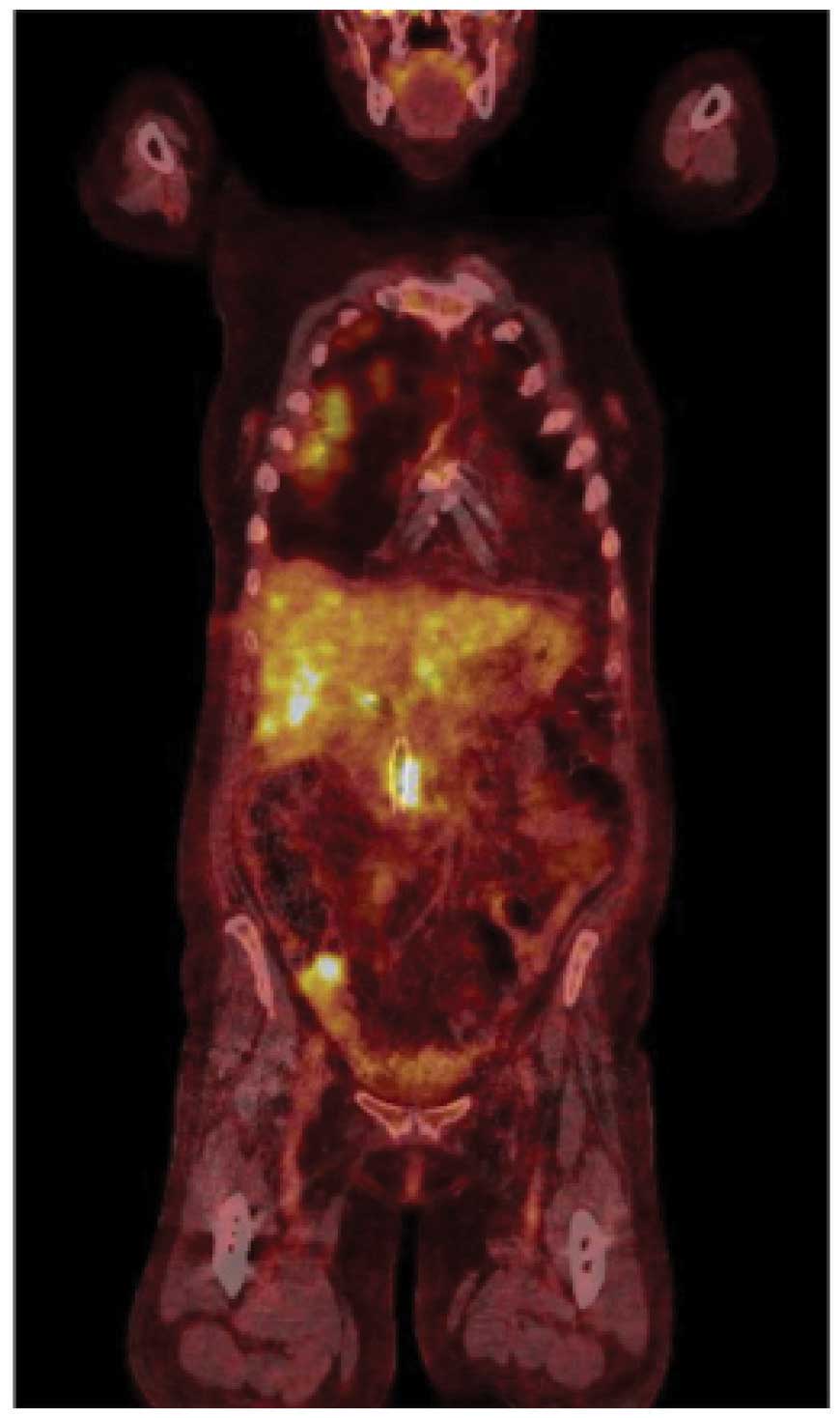
diameter (Fig. 1). Whole-body
18F-fluorodeoxyglucose positron emission tomography was performed,
which revealed multiple metastases to liver, spine, retroperitoneal
lymph nodes and lungs (Fig. 2).
Histopathological analysis
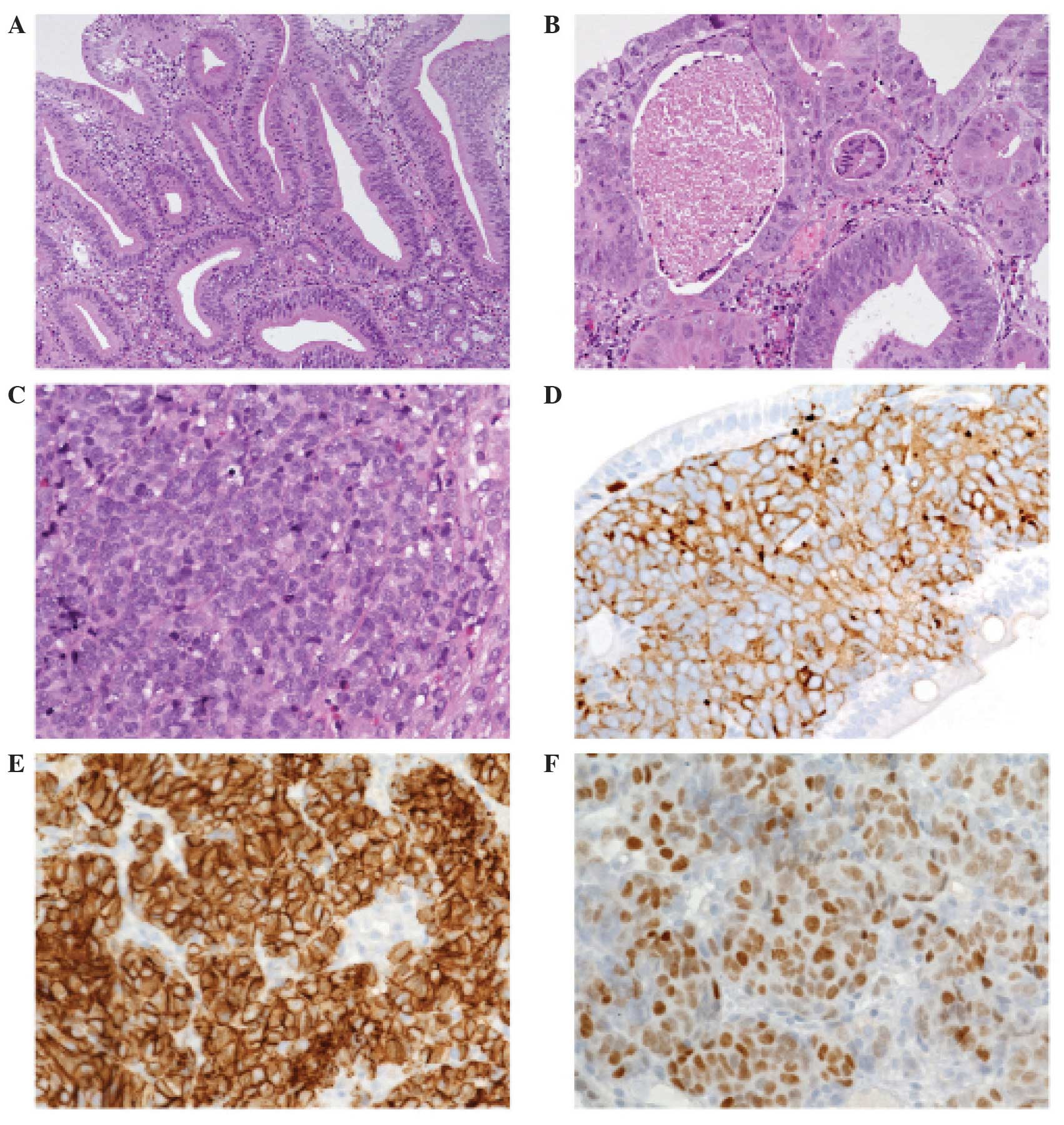
Biopsy material of the ampullary lesion disclosed a
complex malignant epithelial lesion with a non-invasive adenoma
component progressing into invasive intestinal-type adenocarcinoma.
Other parts of the lesion consisted of typical neuroendocrine small
cell carcinoma with diffuse growth pattern, composed of small to
medium sized cells with minimal cytoplasm and fusiform nuclei with
finely granular chromatin and inconspicuous nucleoli (Fig. 3). The mitotic rate was high and
necrosis occurred frequently. Upon immunohistochemical staining,
the tumour was positive for neuroendocrine markers, including
chromogranin A, synaptophysin and cluster of differentiation
(CD)56/neural cell adhesion molecule (NCAM). The intestinal
transcription factor, caudal type homeobox (CDX)-2, was positive
for both exocrine and endocrine tumour components (Fig. 3). Based upon these findings, a
diagnosis of MANEC of the ampulla was made.
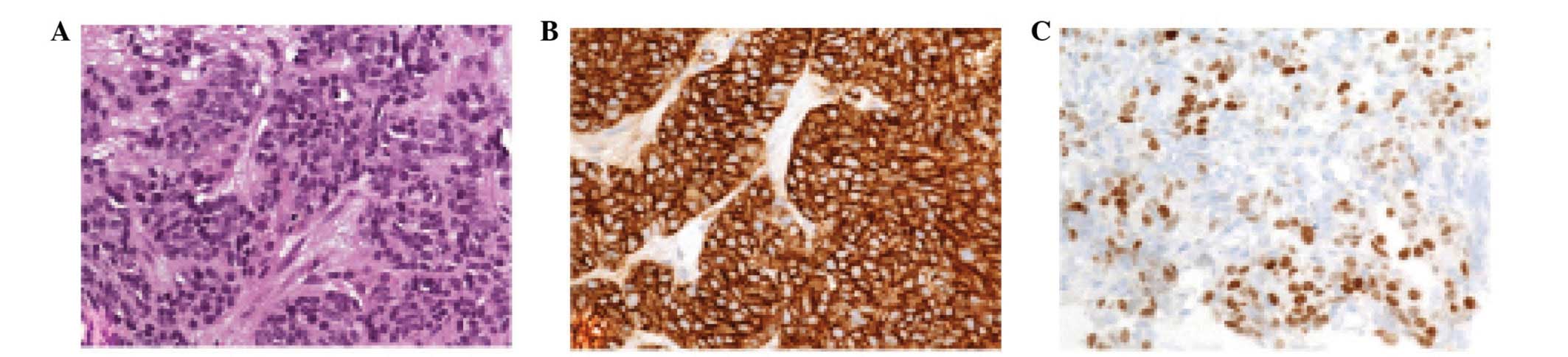
Histology obtained from a liver lesion revealed pure
neuroendocrine small cell cancer, which was positive for
neuroendocrine markers and for CDX-2, thereby proving its
gastrointestinal/ampullary origin (Fig.
4).
Discussion
The first description of a gastrointestinal tumour
exhibiting both exocrine and neuroendocrine differentiation was
published in 1924 by Cordier (13).
The widespread use of immunohistochemical techniques in the last
decades has led to the recognition that neuroendocrine cells are
present rather frequently in exocrine malignancies of the
gastrointestinal tract (14). True
mixed tumour types, including MANECs, are relatively uncommon,
occurring predominantly in the stomach and colorectum.
According to the World Health Organisation
definition, MANECs are morphologically recognisable as both
gland-forming and neuroendocrine neoplasms with an arbitrary
requirement of at least 30% of either component (15). They are defined as carcinomas since
both components are histologically malignant. MANECs must be
differentiated from collision tumour types (16) or so-called amphicrine tumour types
(17), which are characterized by
usually monomorphic growth of cells exhibiting both exocrine and
endocrine differentiation.
Patients with gastrointestinal MANECs appear to
exhibit an improved median overall survival compared with patients
with pure NECs, and this finding may be associated with the higher
stage at the time of diagnosis of the latter (14). In a previous investigation of
colorectal MANECs, no different patient survival was observed
between NECs and MANECs (18),
suggesting that certain clinical differences between NECs and
MANECs may be site-associated (19).
In general, MANECs are highly aggressive tumour types with a high
risk for distant metastasis, and prognosis is often dismal. It is
largely unclear whether the glandular or the neuroendocrine
component is the major driving force of disease progression and
thus crucial for metastatic cancer spread (11,20,21).
In oncological practice, distant metastases are not
normally assessed by histology, when the responsible primary tumour
is known (22). The presented case
exhibited widespread dissemination of the neuroendocrine small cell
carcinoma, however, not of the adenocarcinoma component with
potentially severe implications for the choice of chemotherapy. The
present study determined that the time has come to reconsider the
indication for testing metastatic sites in standard oncology
practice.
In conclusion, the present report presented a rare
case of a MANEC originating from the ampulla of Vater. The tumour
tissue from the present patient was biopsied and assessed at both
primary and metastatic sites, proving discordant results. As
exemplified by this true mixed tumour type, tumour heterogeneity
evolves as the major challenge in oncology today. The assessment of
metastatic sites may render valuable diagnostic information that is
crucial for clinical decision-making and patient management.
References
|
1
|
Albores-Saavedra J, Hruban RH, Klimstra DS
and Zamboni G: Invasive adenocarcinoma of the ampullary region.
Bosman FT, Carneiro F, Hruban RH and Theise ND: World health
organization classification of tumours of the digestive system.
IARC Press. (Lyon). 87–91. 2010.
|
|
2
|
Kumari N, Prabha K, Singh RK, Baitha DK
and Krishnani N: Intestinal and pancreatobiliary differentiation in
periampullary carcinoma: The role of immunohistochemistry. Hum
Pathol. 44:2213–2219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ang DC, Shia J, Tang LH, Katabi N and
Klimstra DS: The utility of immunohistochemistry in subtyping
adenocarcinoma of the ampulla of vater. Am J Surg Pathol.
38:1371–1379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter JT, Grenert JP, Rubenstein L,
Stewart L and Way LW: Tumors of the ampulla of vater:
Histopathologic classification and predictors of survival. J Am
Coll Surg. 207:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WS, Choi DW, Choi SH, Heo JS, You DD
and Lee HG: Clinical significance of pathologic subtype in
curatively resected ampulla of vater cancer. J Surg Oncol.
105:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robert PE, Leux C, Ouaissi M, Miguet M,
Paye F, Merdrignac A, Delpero JR, Schwarz L, Carrere N, Muscari F,
et al: Predictors of long-term survival following resection for
ampullary carcinoma: A large retrospective French multicentric
study. Pancreas. 43:692–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nassar H, Albores-Saavedra J and Klimstra
DS: High-grade neuroendocrine carcinoma of the ampulla of vater: A
clinicopathologic and immunohistochemical analysis of 14 cases. Am
J Surg Pathol. 29:588–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carter JT, Grenert JP, Rubenstein L,
Stewart L and Way LW: Neuroendocrine tumors of the ampulla of
Vater: Biological behavior and surgical management. Arch Surg.
144:527–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moncur JT, Lacy BE and Longnecker DS:
Mixed acinar-endocrine carcinoma arising in the ampulla of Vater.
Hum Pathol. 33:449–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Musialik JA, Kohut MJ, Marek T, Wodołazski
A and Hartleb M: Composite neuroendocrine and adenomatous carcinoma
of the papilla of Vater. World J Gastroenterol. 15:4199–4200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L and DeMay RM: Cytological features
of mixed adenoneuroendocrine carcinoma of the ampulla: Two case
reports with review of literature. Diagn Cytopathol. 42:1075–1084.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Z, Xiao WD, Li Y, Huang S, Cai J and
Ao J: Mixed adenoneuroendocrine carcinoma of the ampulla: Two case
reports. World J Gastroenterol. 21:2254–2259. 2015.PubMed/NCBI
|
|
13
|
Cordier R: Les cellules argentaffines dans
les tumeurs intestinales. Arch Int Med Exp. 1:59–63. 1924.
|
|
14
|
La Rosa S, Marando A, Sessa F and Capella
C: Mixed adenoneuroendocrine carcinomas (MANECs) of the
gastrointestinal tract: An update. Cancers (Basel). 4:11–30. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra DS, Klöppel Komminoth P and Solcia E: Nomenclature and
classification of neuroendocrine neoplasms of the digestive system.
Bosman FT, Carneiro F, Hruban RH and Theise ND: World health
organization classification of tumours of the digestive system.
IARC Press. (Lyon). 13–14. 2010.
|
|
16
|
Khurana A, Sharma A, Gupta G and Gandhi
JS: Peri-ampullary collision tumor-high grade neuroendocrine
carcinoma and signet ring cell carcinoma: A case report and review
of literature. Indian J Pathol Microbiol. 54:161–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ginori A, Lo Bello G, Vassallo L and
Tripodi SA: Amphicrine carcinoma of the ampullary region. Tumori.
101:e70–e72. 2015.PubMed/NCBI
|
|
18
|
La Rosa S, Marando A, Furlan D, Sahnane N
and Capella C: Colorectal poorly differentiated neuroendocrine
carcinomas and mixed adenoneuroendocrine carcinomas: Insights into
the diagnostic immunophenotype, assessment of methylation profile
and search for prognostic markers. Am J Surg Pathol. 36:601–611.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klöppel G, Rindi G, Anlauf M, Perren A and
Komminoth P: Site-specific biology and pathology of
gastroenteropancreatic neuroendocrine tumors. Virchows Arch.
451(Suppl 1): S9–S27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harada K, Sato Y, Ikeda H, Maylee H,
Igarashi S, Okamura A, Masuda S and Nakanuma Y: Clinicopathologic
study of mixed adenoneuroendocrine carcinomas of hepatobiliary
organs. Virchows Arch. 460:281–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurzu S, Kadar Z, Bara T, Bara T Jr,
Tamasi A, Azamfirei L and Jung I: Mixed adenoneuroendocrine
carcinoma of gastrointestinal tract: Report of two cases. World J
Gastroenterol. 21:1329–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Krieken JH, Jung A, Kirchner T,
Carneiro F, Seruca R, Bosman FT, Quirke P, Fléjou JF, Plato Hansen
T, de Hertogh G, et al: KRAS mutation testing for predicting
response to anti-EGFR therapy for colorectal carcinoma: Proposal
for an European quality assurance program. Virchows Arch.
453:417–431. 2008. View Article : Google Scholar : PubMed/NCBI
|