Introduction
Gemtuzumab ozogamicin (GO) is a recombinant
humanized immunoglobulin (Ig)G4 anti-cluster of differentiation
(CD)33 monoclonal antibody conjugated to N-acetyl-γ calicheamicin
dimethylhydrazide, a naturally potent antibiotic. It has been
introduced for the treatment of acute promyelocytic leukemia (APL),
since large quantities of CD33 are commonly expressed on the
surface of APL cells. Although several previous studies have
reported successful results for the use of GO as APL therapy
(1–8),
no large clinical studies of GO for APL treatment have been
performed. APL is characterized by fibrinolytic-type disseminated
intravascular coagulation (DIC). The present study reported two
patients who developed DIC following treatment with GO, although
their coagulation profiles revealed no presence of DIC prior to the
treatment. Since prominent DIC was transiently observed following
treatment with GO, it may be an adverse event caused by GO. Very
limited information exists regarding DIC occurring following
treatment with GO (9), and its
mechanism remains unclear. The present study used recombinant human
soluble thrombomodulin (rTM) for the treatment of DIC, although rTM
in combination with GO has not been previously reported, to the
best of our knowledge. The present study reported on these two
patients with relapsed/refractory APL who exhibited DIC following
treatment with GO, receiving rTM therapy for DIC.
Case reports
Case 1
An 85-year-old man presented with pancytopenia in
August 2011 at the First Department of Internal Medicine, Kansai
Medical University (Osaka, Japan). A chromosome analysis revealed
46, XY, t(15;17) (q22;21), and APL was diagnosed. Treatment with
all-trans retinoic acid (ATRA) and chemotherapy, including
idarubicin (IDA), was administered, and hematological complete
remission (CR) was attained in November 2011. The patient received
maintenance chemotherapy, according to the PETHEMA LPA 2005 regimen
(10), and achieved molecular CR in
December 2011. The patient continued to receive ATRA until he
developed interstitial pneumonia in April 2012. In October 2012,
the patient had a molecular relapse and restarted ATRA, however,
the disease was refractory. In December 2012, the patient received
arsenic trioxide (ATO) and achieved a second CR. In October 2013,
the patient had a third molecular relapse. A bone marrow smear
revealed 1.8% APL cells. The mRNA of promyelocytic leukemia
(PML)/retinoic acid receptor (RAR)α was detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR;
1.8×104 copies/µg). The results of laboratory tests were
as follows: Erythrocyte count, 3.93×1012/l; platelet
count, 15.1×109/l; leukocyte count, 5.9×109/l
(the leukocytes included 0% promyelocytes, 4.5% monocytes, 45.5%
neutrophils and 34.5% lymphocytes). GO (9 mg/m2) was
administered on days 1 and 15. The clinical course is shown in
Fig. 1. Prominent DIC was transiently
observed following the treatment. On day 4, the prothrombin time
(PT) was 92.6%, the activated partial thromboplastin time (APTT)
was 26.6 sec, the fibrinogen level was 391 g/l, the fibrin
degradation product (FDP) level was 149.8 µg/ml and the fibrin
degradation product D-dimer (FDP-DD) level was elevated to 92.6
µg/ml. Treatment with rTM was initiated. During this treatment, the
FDP and FDP-DD levels gradually decreased. A reduction in the level
of fibrinogen was not observed. GO was administered again on day
15. This caused a slight increase in FDP-DD levels, which promptly
decreased. Molecular CR was confirmed on day 41.
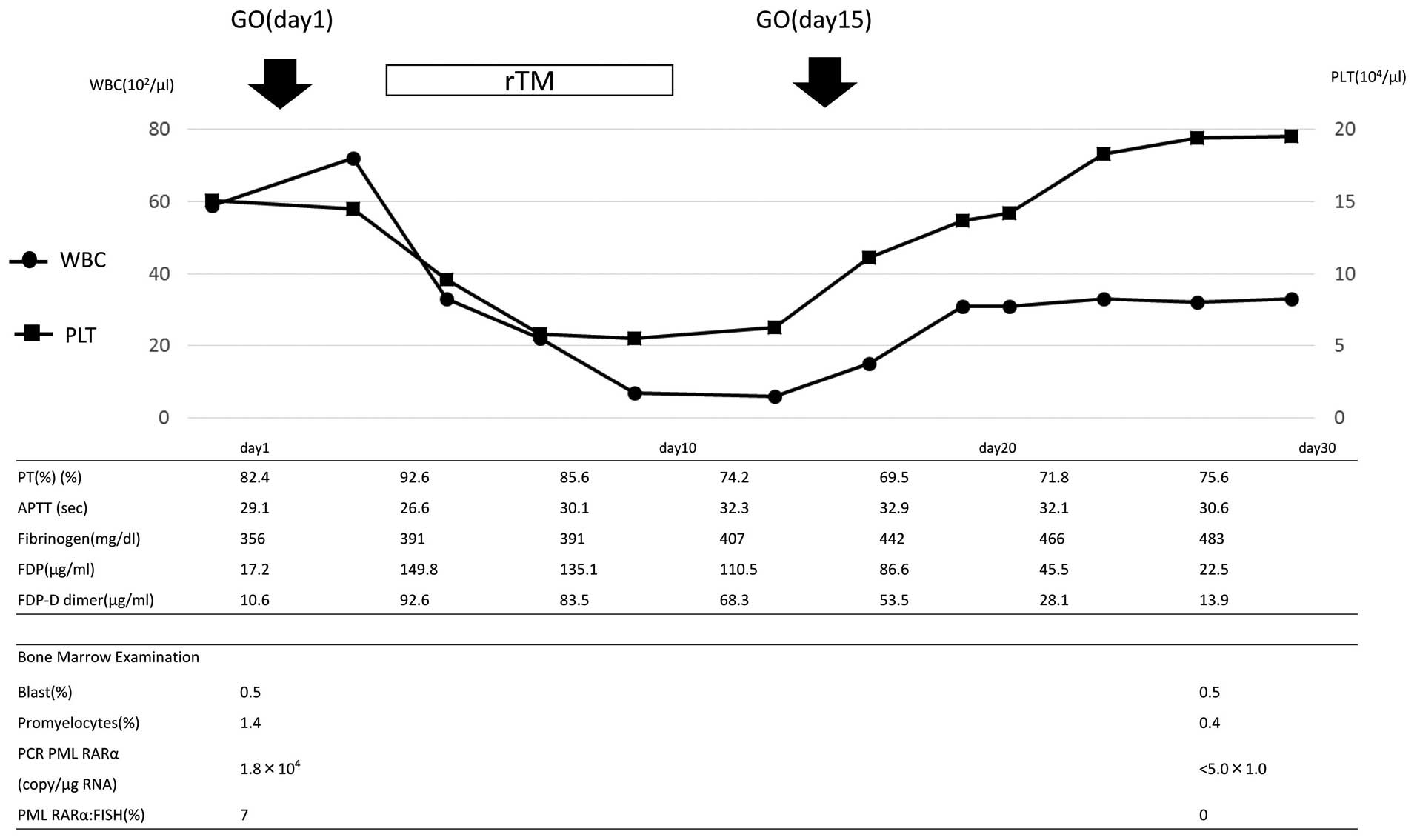 | Figure 1.Clinical course of the patient in case
1. GO, gemtuzumab ozogamicin; PT, prothrombin time; WBC, white
blood cell count; PLT, platelets; rTM, recombinant human soluble
thrombomodulin; APTT, activated partial thromboplastin time; FDP,
fibrin degradation product; PCR, polymerase chain reaction; PML,
promyelocytic leukemia; RAR, retinoic acid receptor; FISH,
fluorescent in situ hybridization. |
Case 2
An 80-year-old man was diagnosed with APL in 2004 at
the First Department of Internal Medicine, Kansai Medical
University. The patient was administered treatment with ATRA plus
IDA, and CR was attained. The patient relapsed in June 2012. A
second treatment with ATRA was administered and the patient
exhibited a second CR. A second relapse occurred and the patient
received ATO, however, this was subsequently discontinued due to QT
interval prolongation. Tamibarotene was started, however, the
disease was resistant. A bone marrow smear revealed 22% APL cells.
The mRNA of PML/RARα was detected by RT-qPCR (8.3×104
copies/µg). The results of laboratory tests were as follows:
Erythrocyte count, 2.43×1012/l; platelet count,
10.3×109/l; leukocyte count, 9.0×109/l (the
leukocytes included 0% promyelocytes, 1% monocytes, 55% neutrophils
and 43% lymphocytes).
GO (9 mg/m2) was administered on day 1.
The clinical course is shown in Fig.
2. Although the coagulation profile revealed no presence of DIC
prior to the treatment with GO, prominent DIC was transiently
observed following the treatment. On day 4, the PT was 77.6%, the
APTT was 29.4 sec, the fibrinogen level was 350 g/l, the FDP level
was 176.4 µg/ml and the FDP-DD level was elevated to 109 µg/ml. rTM
treatment was initiated, and the FDP and FDP-DD level gradually
decreased. On day 9, the patient acquired a severe infection, which
transiently increased the FDP-DD level. Following recovery from the
infection, the FDP-DD level returned to normal. During the
treatment course, reduction of the fibrinogen level was not
observed. Due to the infection, the patient was unable to receive
GO on day 15. Molecular CR was confirmed on day 24.
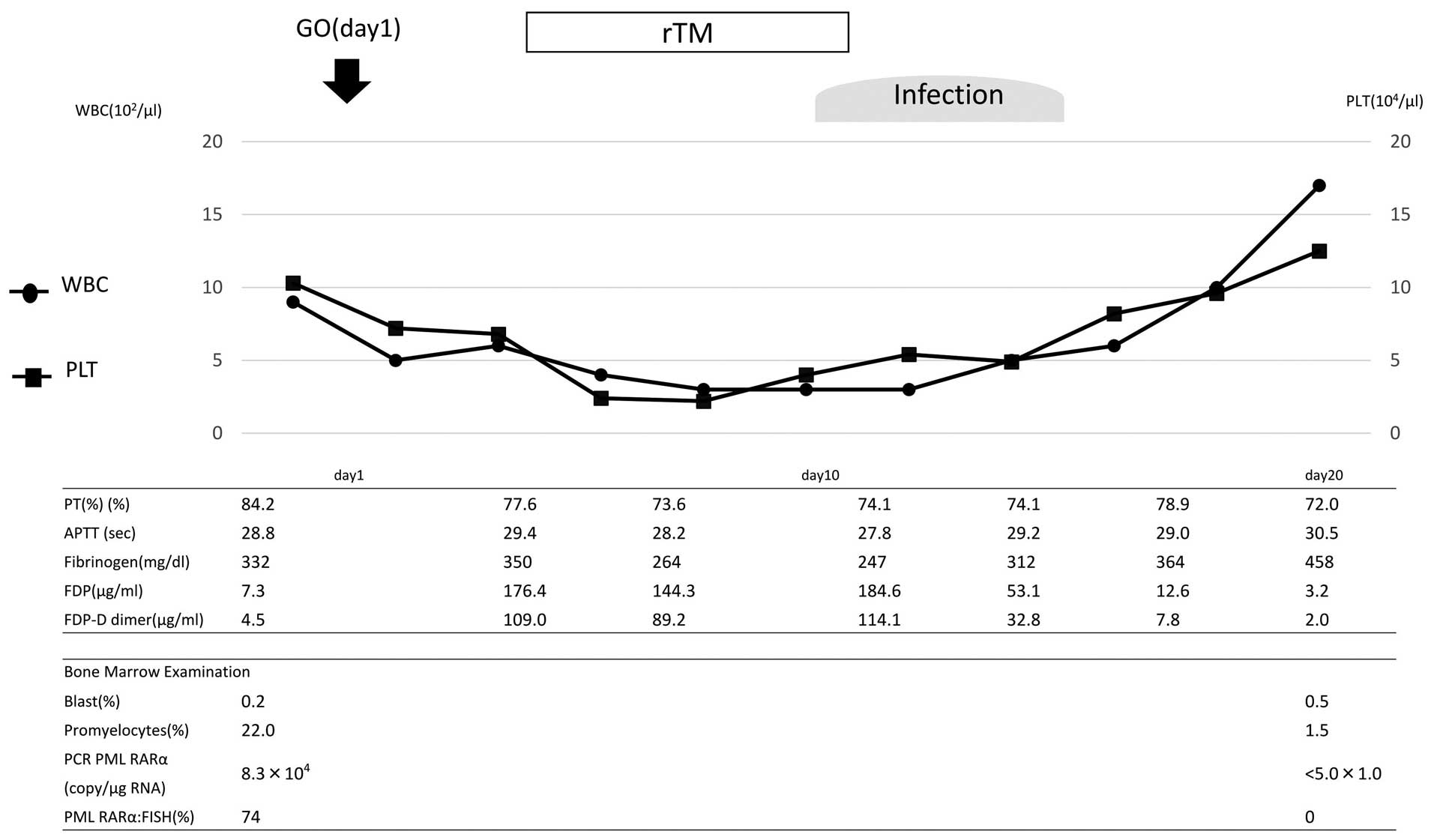 | Figure 2.Clinical course of the patient in case
2. GO, gemtuzumab ozogamicin; PT, prothrombin time; WBC, white
blood cell count; PLT, platelets; rTM, recombinant human soluble
thrombomodulin; APTT, activated partial thromboplastin time; FDP,
fibrin degradation product; PCR, polymerase chain reaction; PML,
promyelocytic leukemia; RAR, retinoic acid receptor; FISH,
fluorescent in situ hybridization. |
Discussion
GO is a recombinant humanized IgG4 anti-CD33
monoclonal antibody conjugated to N-acetyl-γ calicheamicin
dimethylhydrazide, a naturally potent antibiotic. It has been
introduced for the treatment of APL (1–8), although
its efficacy in ATRA- and chemotherapy-resistant fully-relapsed APL
remains to be elucidated. Certain basic reasons may explain the
effectiveness of GO for the treatment of APL. Firstly, large
quantities of CD33 are commonly expressed on the surface of APL
cells. Secondly, the levels of P-glycoprotein (a
multidrug-resistant glycoprotein) are lower on the surface of APL
cells compared with on the surface of acute myeloid leukemia (AML)
cells. Thirdly, APL cells are highly sensitive to free
calicheamicin, an antitumor antibiotic (2,3).
Several previous studies have hypothesized the
efficacy of GO for APL (1,5). In a previous study performed at the MD
Anderson Cancer Center (Houston, TX, USA), the CR rate was 81% in
high-risk patients who received GO (5). The combination of ATRA and ATO plus GO
is now being evaluated in a North American Intergroup APL trial for
high-risk APL. Italian investigators noted that early treatment of
molecular relapse of APL with single-agent GO resulted in longer
survival compared with the initiation of treatment at hematological
relapse (1).
The relative rarity of APL, particularly molecular
relapse of APL, makes a randomized study to confirm these findings
infeasible. The SWOG106 study, comprising a trial of GO with
cytarabine plus anthracycline in relapsed AML, found no improvement
in event-free survival, disease-free survival or overall survival
(11). These results prompted Pfizer
to voluntarily withdraw GO from the market in the United States in
June 2010, prior to the results of other randomized trials being
made available.
A post-marketing study of GO in Japan reported that
the frequencies of adverse events were myelosuppression (67%),
infusion reaction (47%), liver dysfunction (43%), infection (35%),
bleeding (17%) and DIC (7%). Prominent DIC following treatment with
GO was reported in two previous cases (9). In that previous study, the investigators
assumed that GO may increase the frequency and severity of DIC due
to APL cells collapsing rapidly. However, the frequency of DIC in
the post-marketing surveillance was 7%, meaning that DIC does not
necessarily occur in all cases. If DIC arose through the collapse
of APL cells, it may be proportional to the quantity of APL cells.
In the present study, case 1 exhibited a molecular relapse,
however, the number of APL cells was so small that it could not be
counted in the bone marrow smear and blood tests. Therefore, the
quantity of tumor cells must have been small, however, DIC still
occurred. By contrast, following the treatment with GO on day 15,
DIC did not occur. From this clinical course, it can be assumed
that DIC must have occurred through a mechanism other than the
quantity of APL cells. The present study hypothesized that GO may
damage vascular endothelial cells and cause DIC, since GO is said
to be associated with the development of sinusoidal obstruction.
The mechanism of sinusoidal obstruction caused by GO remains
unclear, however, it may be similar to DIC. Since the initial onset
of DIC and sinusoidal obstruction is vascular endothelial damage,
it was assumed that rTM may prevent DIC from occurring in the
administration of 15 days of GO. The present study initiated rTM on
day 4, which was soon after DIC occurred and continued it until day
11, then administered GO on day 15. The present study hypothesized
that the effects of rTM remained on day 15 and assisted with the
treatment of endothelial damage, therefore, the increase of FDP-DD
was less than on day 1.
APL is characterized by fibrinolytic-type DIC, which
is characterized by increasing levels of FDP-DD and decreasing
levels of fibrinogen. rTM was approved for treatment of DIC in
Japan in 2008. Previously, heparin and gabexate mesilate were used
prophylactically prior to the treatment with GO (9). In the present study, rTM was used for
the first time, to the best of our knowledge, for DIC triggered by
GO. Although a report indicated that rTM was effective in the
treatment with ATRA or ATO for DIC (12), its usefulness for treatment of APL has
not been confirmed in larger studies. No bleeding-associated
mortality was noted during induction chemotherapy in APL patients
who received rTM (13). Notably,
severe hemorrhage requiring red blood cell transfusion at the time
of diagnosis of APL was reduced following the initiation of rTM
(14). Use of rTM in combination with
ATO for relapse of APL promptly improved DIC without any adverse
effects in one previous case report (15). An in vitro study demonstrated
that exposure of APL cells to rTM significantly downregulated the
level of annexin II, resulting in a decrease in plasmin production
(16). However, the mechanism remains
to be elucidated in vivo.
rTM was stopped after 7 days, since its safety for
use over 7 days remains to be determined. In the present case,
following treatment with rTM, the efficacy of rTM remained and
FDP-DD kept decreasing.
Each of the cases in the present study achieved
early molecular CR following treatment with GO. Therefore, GO may
be a promising agent for the management of APL and may become one
of the treatment options for recurrent APL in elderly patients.
Since DIC is the most serious adverse event of GO treatment,
elucidation of its mechanism and establishment of a treatment
strategy are warranted.
References
|
1
|
Lo-Coco F, Cimino G, Breccia M, Noguera
NI, Diverio D, Finolezzi E, Pogliani EM, Di Bona E, Micalizzi C,
Kropp M, et al: Gemtuzumab ozogamicin (Mylotarg) as a single agent
for molecularly relapsed acute promyelocytic leukemia. Blood.
104:1995–1999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeshita A, Shinjo K, Naito K, Matsui H,
Sahara N, Shigeno K, Horii T, Shirai N, Maekawa M, Ohnishi K, et
al: Efficacy of gemtuzumab ozogamicin on ATRA- and
arsenic-resistant acute promyelocytic leukemia (APL) cells.
Leukemia. 19:1306–1311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aribi A, Kantarjian HM, Estey EH, Koller
CA, Thomas DA, Kornblau SM, Faderl SH, Laddie NM, Garcia-Manero G
and Cortes JE: Combination therapy with arsenic trioxide, all-trans
retinoic acid, and gemtuzumab ozogamicin in recurrent acute
promyelocytic leukemia. Cancer. 109:1355–1359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanz MA, Montesinos P, Rayón C, Holowiecka
A, de la Serna J, Milone G, de Lisa E, Brunet S, Rubio V, Ribera
JM, et al: Risk-adapted treatment of acute promyelocytic leukemia
based on all-trans retinoic acid and anthracycline with addition of
cytarabine in consolidation therapy for high-risk patients: Further
improvements in treatment outcome. Blood. 115:5137–5146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ravandi F, Estey E, Jones D, Faderl S,
O'Brien S, Fiorentino J, Pierce S, Blamble D, Estrov Z, Wierda W,
et al: Effective treatment of acute promyelocytic leukemia with
all-trans-retinoic acid, arsenic trioxide and gemtuzumab
ozogamicin. J Clin Oncol. 27:504–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ravandi F, Estey EH, Appelbaum FR, Lo-Coco
F, Schiffer CA, Larson RA, Burnett AK and Kantarjian HM: Gemtuzumab
ozogamicin: Time to resurrect? J Clin Oncol. 30:3921–3923. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo-Coco F, Latagliata R and Breccia M:
Management of acute promyelocytic leukemia in the elderly. Mediterr
J Hematol Infect Dis. 5:e20130452013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeshita A: Efficacy and resistance of
gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol.
97:703–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeshita A, Shinjo K, Naito K, Matsui H,
Sahara N, Shigeno K, Suzumura T, Horii T, Shirai N, Maekawa M, et
al: Two patients with all-trans retinoic acid-resistant acute
promyelocytic leukemia treated successfully with gemtuzumab
ozogamicin as a single agent. Int J Hematol. 82:445–448. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanz MA, Martín G, González M, León A,
Rayón C, Rivas C, Colomer D, Amutio E, Capote FJ, Milone GA, et al:
Risk-adapted treatment of acute promyelocytic leukemia with
all-trans-retinoic acid and anthracycline monochemotherapy: A
multicenter study by the PETHEMA group. Blood. 103:1237–1243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petersdorf SH, Kopecky KJ, Slovak M,
Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ,
Stuart RK, Walter RB, Tallman MS, Stenke L and Appelbaum FR: A
phase 3 study of gemtuzumab ozogamicin during induction and
postconsolidation therapy in younger patients with acute myeloid
leukemia. Blood. 121:4854–4860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikezoe T: Pathogenesis of disseminated
intravascular coagulation in patients with acute promyelocytic
leukemia, and its treatment using recombinant human soluble
thrombomodulin. Int J Hematol. 100:27–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikezoe T, Takeuchi A, Isaka M, Arakawa Y,
Iwabu N, Kin T, Anabuki K, Sakai M, Taniguchi A, Togitani K and
Yokoyama A: Recombinant human soluble thrombomodulin safely and
effectively rescues acute promyelocytic leukemia patients from
disseminated intravascular coagulation. Leuk Res. 36:1398–1402.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawano N, Kuriyama T, Yoshida S, Yamashita
K, Ochiai H, Nakazaki S, Tasaki A and Ueda A: Clinical features and
treatment outcomes of six patients with disseminated intravascular
coagulation resulting from acute promyelocytic leukemia and treated
with recombinant human soluble thrombomodulin at a single
institution. Intern Med. 52:55–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shindo M, Ikuta K, Addo L, Ito S, Fujiya
M, Torimoto Y and Kohgo Y: Successful control of disseminated
intravascular coagulation by recombinant thrombomodulin during
arsenic trioxide treatment in relapsed patient with acute
promyelocytic leukemia. Case Rep Hematol.
2012:9081962012.PubMed/NCBI
|
|
16
|
Ikezoe T, Yang J, Nishioka C, Isaka M,
Iwabu N, Sakai M, Taniguchi A, Honda G and Yokoyama A:
Thrombomodulin enhances the antifibrinolytic and antileukemic
effects of all-trans retinoic acid in acute promyelocytic leukemia
cells. Exp Hematol. 40:457–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
















