Introduction
The echinoderm microtubule-associated protein-like
4-ALK (EML4-ALK) fusion gene is a known oncogenic driver in
non-small-cell lung cancer (NSCLC), with ALK-translocated NSCLCs
accounting for ~5% of all NSCLCs and 20% of cases in never-smokers
(1). This fusion gene is also
associated with younger age and the adenocarcinoma subtype of
NSCLC. As cases with squamous cell carcinoma of the lung harboring
ALK gene rearrangement are extremely rare (2), its molecular analysis is not routinely
recommended by the National Comprehensive Cancer Network (NCCN)
guideline for the treatment of NSCLC (version 2, 2013) (https://www.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf).
We herein describe a rare case of an elderly male smoker with
squamous cell carcinoma of the lung harboring ALK
rearrangement.
Case presentation
A 76-year-old Japanese man was referred to Yao
Municipal Hospital due to dyspnea and weight loss. The patient
required oxygen supplementation. The past medical history was
unremarkable, apart from smoking history (20 pack-years). The serum
levels of cytokeratin-19 fragment and carcinoembryonic antigen were
elevated to 3.9 ng/ml (normal, <3.5 ng/m) and 65.8 ng/ml
(normal, <5.0 ng/ml), respectively. A chest radiograph
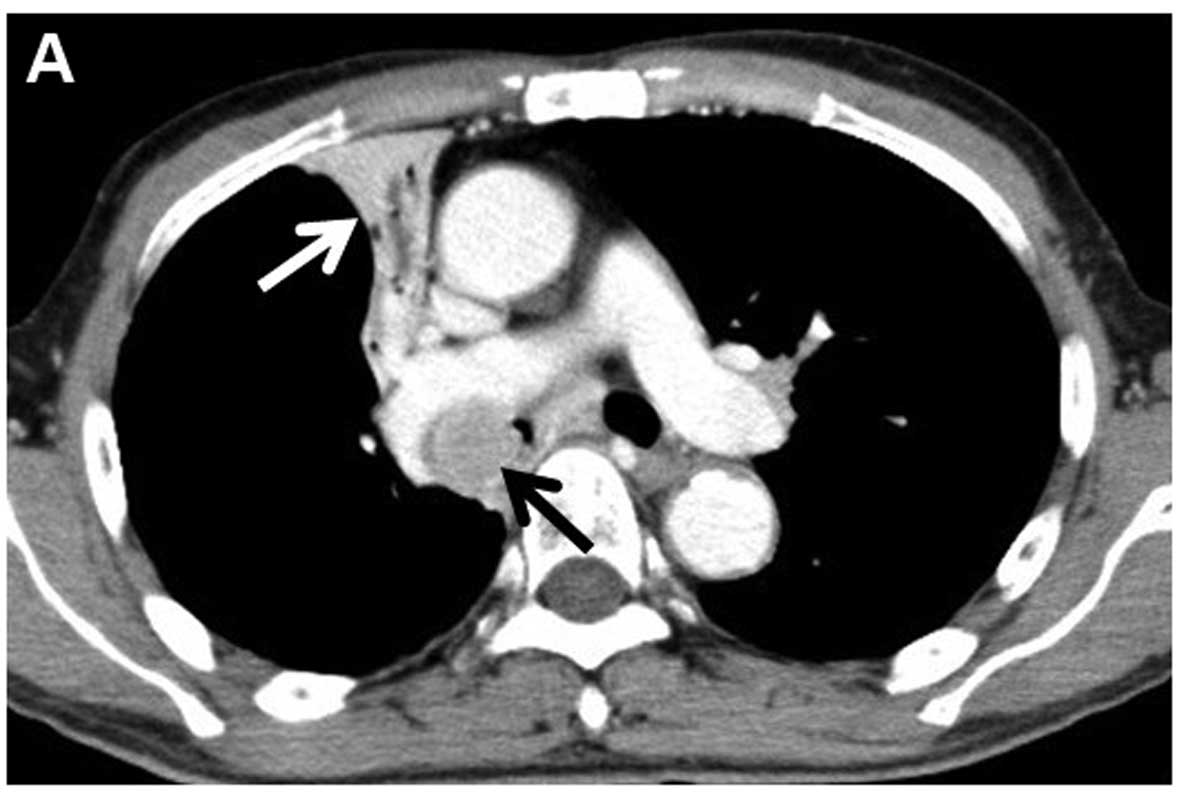
demonstrated right upper lobe atelectasis. Computed tomography (CT)
revealed a 26-mm nodule in the right main bronchus and multiple
enlarged mediastinal lymph nodes (Fig.
1). Magnetic resonance imaging of the brain revealed no brain
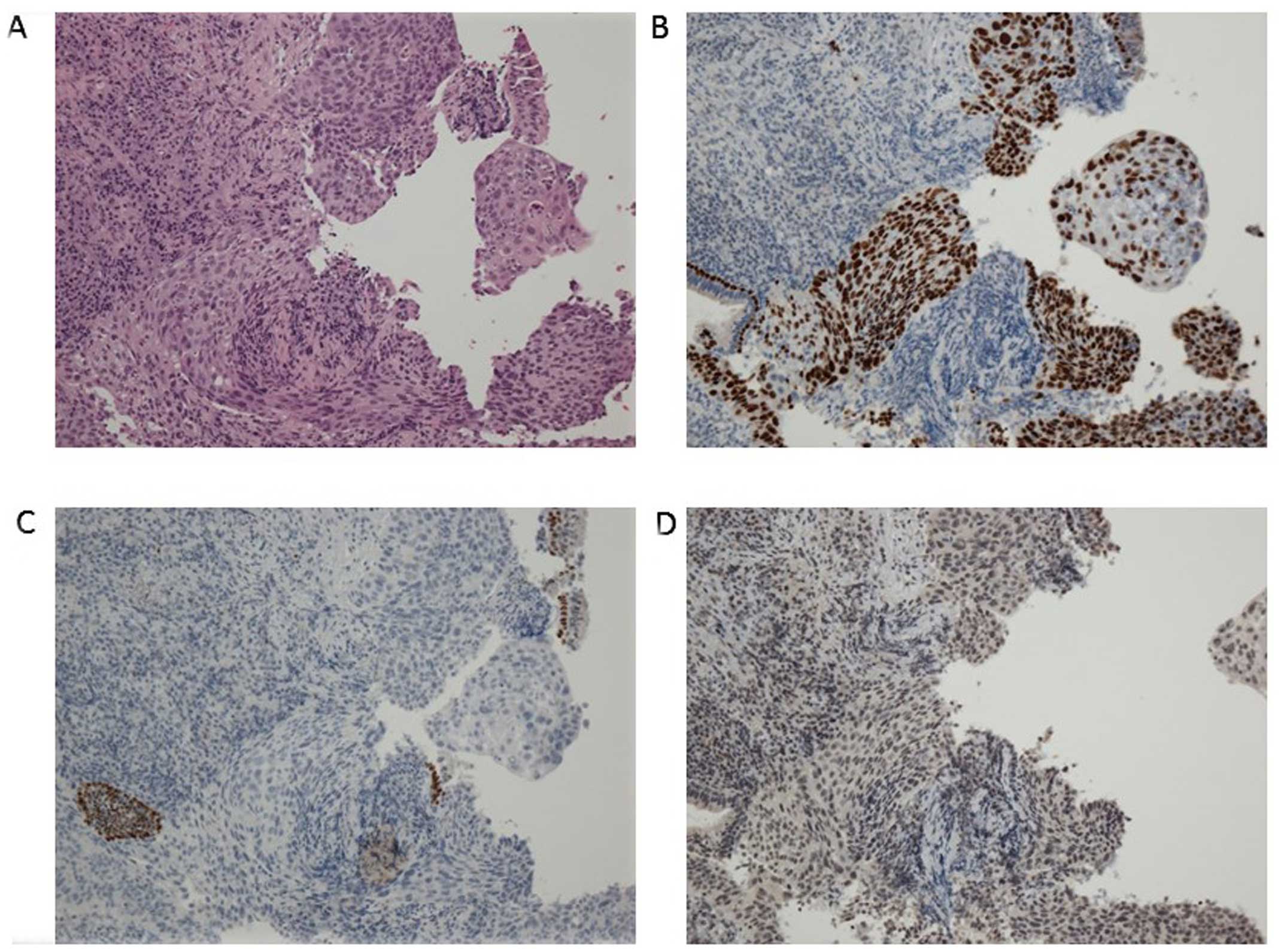
metastasis. A pathological diagnosis of squamous cwell carcinoma
was made using bronchoscopic biopsy (Fig.
2A). There was no component of adenocarcinoma or other
histological type in the biopsy specimen. To further determine the
histological subtype, we performed immunohistochemistry (IHC),
which was positive for p40 and negative for thyroid transcription
factor-1 and napsin A (Fig. 2B–D).
Molecular analysis revealed wild-type epidermal growth factor
receptor gene status. Although ALK testing using IHC (the iAEP
method, ALK detection kit; Nichirei Bioscience, Tokyo, Japan) was
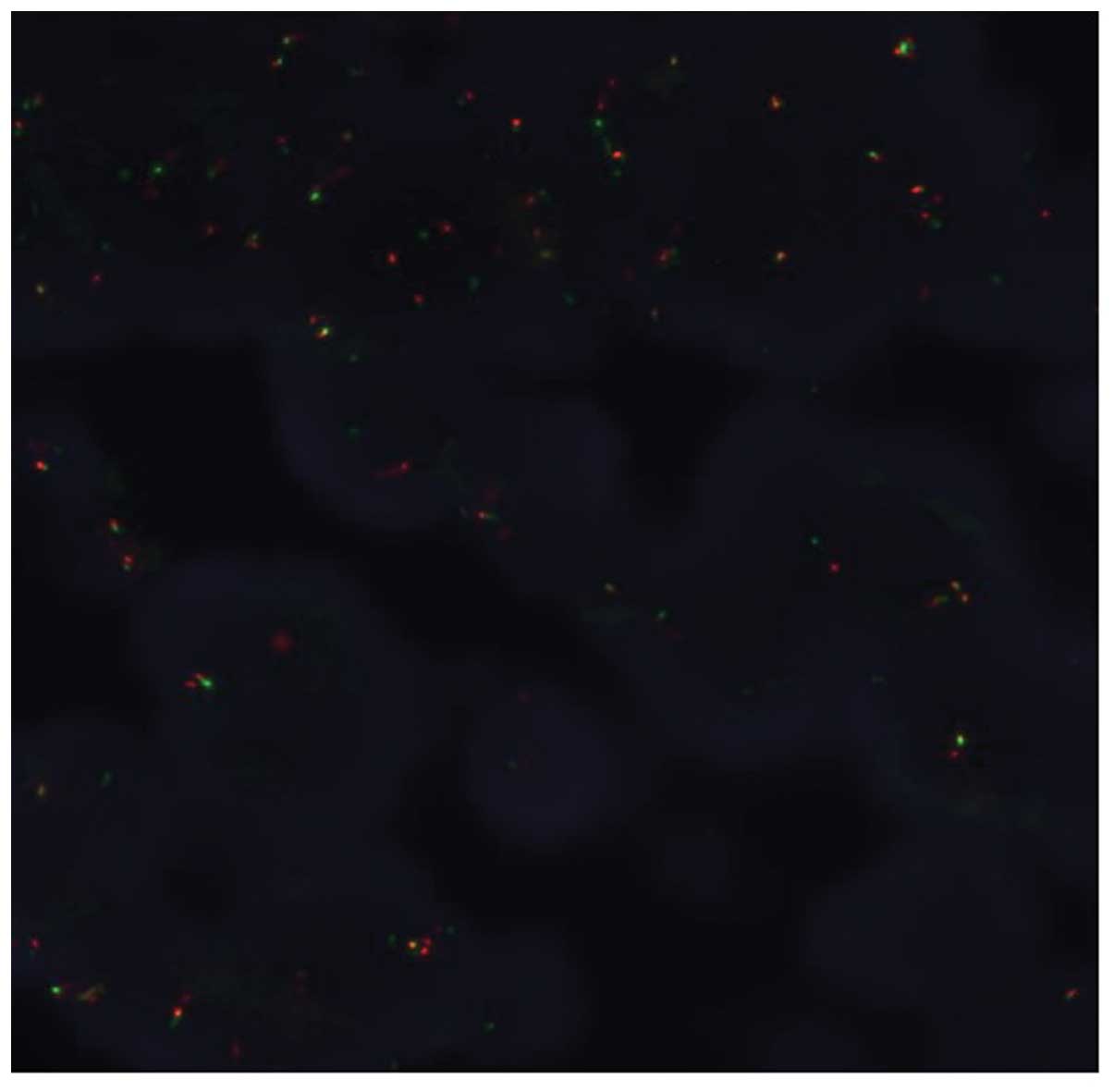
negative, fluorescence in situ hybridization analysis with
break-apart probes for the ALK gene (Vysis Abbott Molecular, Des
Plaines, IL, USA) revealed the presence of an ALK rearrangement
(Fig. 3). Based on these results, the
patient was clinically diagnosed with T3N2M0, stage IIIA squamous
cell carcinoma. The patient had an Easter Cooperative Oncology
Group performance status score of 2, and received radiation therapy
to the primary site and the mediastinum at a dose of 60 Gy in 30
fractions. A CT scan at 4 months post-treatment revealed
improvement of the atelectasis, with a marked decrease in the size
of the tumor. The patient is being regularly followed-up as an
outpatient, without oxygen supplementation.
Discussion
The EML4-ALK fusion gene has been identified as a
potent oncogenic driver in NSCLC. ALK-translocated NSCLCs account
for ~5% of all NSCLCs and 20% of NSCLCs in never-smokers (1). ALK rearrangement has been associated
with several clinicopathological characteristics: Never- or light
smokers, younger age at diagnosis, adenocarcinoma histology, signet
ring cells, and mutual exclusivity from other major driver genes
(1). These clinicopathological
characteristics were not applicable to our case, as the patient was
a current smoker with hilar-type squamous cell carcinoma. ALK
rearrangement in squamous cell carcinoma is extremely rare, with an
estimated prevalence of ALK rearrangement in squamous cell
carcinoma of the lung of only ~0.2–2.5% (2). Thus, its molecular analysis when
excluding adenocarcinoma is not routinely recommended in the NCCN
guideline for the treatment of NSCLC (version 2, 2013).
It is widely known that ALK inhibitors, such as
crizotinib or alectinib, have significantly improved treatment
response among NSCLC patients with ALK rearrangement. The
determination of ALK-positive status is necessary to identify
patients with advanced NSCLC who are most likely to benefit from
targeted therapy with an ALK inhibitor. The gold standard for the
detection of predictive ALK rearrangements is currently break-apart
FISH, as it is able to detect all known ALK rearrangements and was
clinically validated in crizotinib clinical trials (3). However, our case was positive for FISH
with a 20% rearrangement-positive cell rate, but negative on IHC.
As a possible explanation for this mismatch, the distinction of IHC
1+ from IHC 0 may be subjective. Re-testing of IHC is desirable;
however, there was no residual sample for further testing in this
case.
It remains unclear whether ALK-positive squamous
cell carcinoma patients show a marked response to ALK-targeted
therapies, which is generally effective for ALK-positive lung
adenocarcinoma. According to two recent case reports published in
China, two 55-year-old female non-smokers with ALK-positive
squamous cell carcinoma responded to crizotinib (4,5). By
contrast, Tamiya et al reported the case of a 78-year-old
male former smoker with ALK-positive squamous cell carcinoma who
did not respond to alectinib, a second-generation ALK inhibitor
(6), although the tumor cells were
confirmed to be diffusely and strongly positive (3+) for ALK on
IHC, as well as FISH.
Although this mutation is rare in squamous cell
carcinoma, its presence may provide additional treatment options.
Our local policy has been to test all patients with advanced NSCLC
who may benefit from targeted treatment for activating mutations
with sufficient biopsy specimens. In the patient described in this
report, subsequent ALK-targeted treatment may be a viable
option.
In summary, we reported a case of ALK-positive
squamous cell carcinoma of the lung. Oncologist should recognize
that ALK translocation may be present in squamous cell carcinoma of
the lung, for which targeted therapy may be an effective
option.
References
|
1.
|
Shaw AT, Yeap BY, Mino-Knudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Caliò A, Nottegar A, Gilioli E, Bria E,
Pilotto S, Peretti U, Kinspergher S, Simonato F, Pedron S, Knuutila
S, et al: ALK/EML4 fusion gene may be found in pure squamous
carcinoma of the lung. J Thorac Oncol. 9:729–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Teixido C, Karachaliou N, Peg V,
Gimenez-Capitan A and Rosell R: Concordance of IHC, FISH and RT-PCR
for EML4-ALK rearrangements. Transl Lung Cancer Res. 3:70–74.
2014.PubMed/NCBI
|
|
4.
|
Wang Q, He Y, Yang X, Wang Y and Xiao H:
Extraordinary response to crizotinib in a woman with squamous cell
lung cancer after two courses of failed chemotherapy. BMC Pulm Med.
14:832014. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhang Q, Wang J and Zhang S:
ALK-rearranged squamous cell lung cancer: A case report. Int J Cjin
Exp Pathol. 8:2195–2198. 2015.
|
|
6.
|
Tamiya A, Shimizu S and Atagi S: A case of
squamous cell carcinoma harboring an EML4/ALK rearrangement that
was unsuccessfully treated with the ALK inhibitor alectinib. J
Thorac Oncol. 10:e742015. View Article : Google Scholar : PubMed/NCBI
|