Introduction
Although small-cell lung cancer (SCLC) is initially
highly sensitive to chemotherapy and radiotherapy, the majority of
the patients eventually experience disease relapse and their
prognosis is generally poor. To date, topotecan is the only Food
and Drug Administration-approved drug for relapsed or refractory
SCLC. In Japan, amrubicin is also available; however, the overall
treatment options for such patients is limited.
Previous phase II studies have demonstrated that
paclitaxel has antitumor activity in patients with pretreated SCLC
(1). Recently, nanoparticle
albumin-bound (nab)-paclitaxel (Abraxane®; Taiho, Tokyo,
Japan) was developed to improve the therapeutic index of
paclitaxel, and randomized studies have confirmed that
nab-paclitaxel was more effective and exhibited a more favorable
safety profile compared with conventional solvent-based paclitaxel
(2).
In this study, based on this background, we
retrospectively reviewed 9 patients with refractory or relapsed
SCLC who were treated with nab-paclitaxel at Kyoto University
Hospital.
Patients and methods
Patients
Between May, 2013 and February,2015, 64 patients
with thoracic malignancies were treated with nab-paclitaxel. Of
those patients, 8 had thymic tumors, 47 had non-small-cell lung
cancer (NSCLC) and 9 had SCLC. Nab-paclitaxel (100
mg/m2) was administered on days 1, 8, and 15 of a 28-day
cycle. Tumor response was evaluated by using the Response
Evaluation Criteria in Solid Tumors, version 1.1 (3), and adverse events were graded by using
the Common Terminology Criteria for Adverse Events, version 4.0
(http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40;
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40%3E*).
Type of relapse
In this study, sensitive relapse was defined as
patients who responded to initial chemotherapy and developed
disease recurrence >3 months after the completion of
chemotherapy, whereas refractory relapse was defined as patients
who did not respond to initial chemotherapy or developed disease
recurrence within 3 months after the completion of
chemotherapy.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median age of the
patients was 67 years (range, 60–76 years) and our sample included
6 men and 3 women. The performance status was 0 in 4, 1 in 3, and 2
in 2 patients. All the patients had been previously treated with ≥2
lines of chemotherapy prior to receiving nab-paclitaxel (range, 2–9
lines). A total of 5 patients were classified as sensitive relapse
and the remaining 4 as refractory relapse. Three patients had been
previously treated with solvent-based paclitaxel.
 | Table I.Patient characteristics at time of
nab-paclitaxel administration (n=9). |
Table I.
Patient characteristics at time of
nab-paclitaxel administration (n=9).
| Patient | Age (years) | Gender | PS (ECOG) | Disease extent | No. of previous
chemotherapy regimens | No. of nab-paclitaxel
cycles | Sensitivity to
first-line chemotherapy | Previous paclitaxel
treatment |
|---|
| 1 | 69 | Male | 1 | ED | 9 | 4 | Sensitive | Yes |
| 2 | 73 | Male | 0 | LD | 6 | 2 | Sensitive | Yes |
| 3 | 65 | Female | 2 | ED | 2 | 2 | Refractory | No |
| 4 | 68 | Male | 1 | ED | 5 | 4 | Sensitive | No |
| 5 | 76 | Male | 0 | LD | 5 | 4 | Sensitive | Yes |
| 6 | 56 | Female | 1 | ED | 2 | 2 | Sensitive | No |
| 7 | 67 | Male | 0 | ED | 2 | 1 | Refractory | No |
| 8 | 64 | Male | 0 | ED | 2 | 1 | Refractory | No |
| 9 | 60 | Female | 2 | ED | 3 | 1 | Refractory | No |
Response
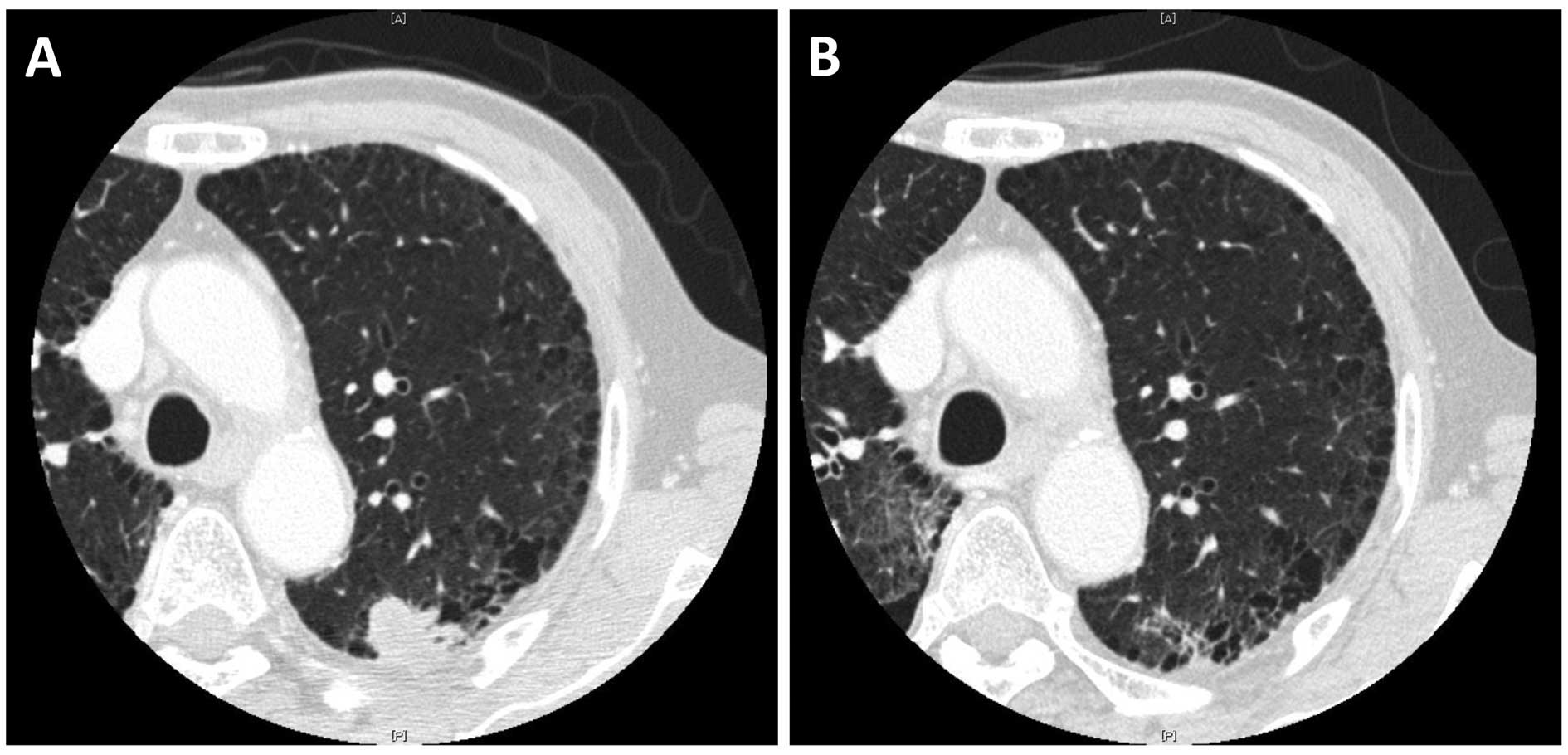
There were 3 partial responses (response rate: 33%)
and a representative case is shown in Fig. 1. Among these 3 patients, all 3 were
classified as sensitive relapse and 2 had been pretreated with
solvent-based paclitaxel, both of whom achieved partial response
after two cycles, but their disease progressed after four cycles of
solvent-based paclitaxel. The median number of cycles of
nab-paclitaxel was 2 (range, 1–4 cycles). The 2 non-evaluable cases
were due to deterioration of epilepsy, without central nervous
system metastases, in one case, and lack of adequate radiographic
follow-up in the other case (Table
II).
 | Table II.Antitumor response. |
Table II.
Antitumor response.
| Type of response | No. of patients | % |
|---|
| Complete
response | 0 | 0 |
| Partial response | 3 | 33 |
| Stable disease | 0 | 0 |
| Progressive
disease | 4 | 45 |
| Not evaluable | 2 | 22 |
| Overall response
rate | 3 | 33 |
Toxicity
The toxicities of nab-paclitaxel are summarized in
Table III. Of the 9 patients, only
1 developed a grade 3 event (leukopenia); no other grade 3/4
adverse events hematological or non-hematological, were observed.
One patient required a dose reduction due to fatigue, but no
treatment discontinuation was required.
 | Table III.Summary of toxicities. |
Table III.
Summary of toxicities.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3/4 (%) |
|---|
| Leukopenia | 1 | 2 | 1 | 0 | 1 (11) |
| Neutropenia | 2 | 1 | 0 | 0 | 0 (0) |
| Anemia | 2 | 3 | 0 | 0 | 0 (0) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 (0) |
| Mucositis | 2 | 0 | 0 | 0 | 0 (0) |
| Nausea | 1 | 0 | 0 | 0 | 0 (0) |
| Vomiting | 0 | 0 | 0 | 0 | 0 (0) |
| Diarrhea | 1 | 0 | 0 | 0 | 0 (0) |
| Constipation | 4 | 0 | 0 | 0 | 0 (0) |
| Fatigue | 6 | 1 | 0 | 0 | 0 (0) |
| Neuropathy | 4 | 1 | 0 | 0 | 0 (0) |
| Myalgia | 1 | 0 | 0 | 0 | 0 (0) |
Discussion
Despite an initial good response to chemotherapy,
almost all SCLC patients experience relapse. To date, a number of
studies on salvage chemotherapy have been conducted, and some
demonstrated a clinical benefit. For example, topotecan prolonged
survival time and improved quality of life compared with supportive
care alone (4), and amrubicin
exhibited efficacy comparable to that of topotecan (5). However, it is clear that the treatment
options remain quite limited. Nab-paclitaxel has been approved for
the treatment of NSCLC, but its efficacy against SCLC remains
unknown.
In this study, we aimed to report the efficacy and
toxicity of nab-paclitaxel for relapsed or refractory SCLC.
Although all the patients were heavily pretreated, 3 patients
achieved partial response with nab-paclitaxel. Interestingly, 2
patients who developed disease progression after weekly
solvent-based paclitaxel treatment responded to nab-paclitaxel. In
in vitro and mouse models, at an equitoxic dose,
nab-paclitaxel-treated groups exhibited more complete regressions,
longer time to recurrence, longer doubling time and prolonged
survival, compared with solvent-based paclitaxel (6). In addtion, at an equal dose,
intratumoral accumulation of paclitaxel was 33% higher for
nab-paclitaxel vs. solvent-based paclitaxel (6). These results were consistent with the
results of clinical trials of breast cancer (2) and NSCLC (7), and our study suggested the superiority
of the nab-paclitaxel to solvent-based paclitaxel in SCLC as
well.
In conclusion, our findings suggest that weekly
administration of nab-paclitaxel may be a useful treatment option
for refractory or relapsed SCLC. However, further investigation of
nab-paclitaxel, alone or in combination with other agents, for SCLC
is warranted.
References
|
1
|
Yamamoto N, Tsurutani J, Yoshimura N, Asai
G, Moriyama A, Nakagawa K, Kudoh S, Takada M, Minato Y and Fukuoka
M: Phase II study of weekly paclitaxel for relapsed and refractory
small cell lung cancer. Anticancer Res. 26:777–781. 2006.PubMed/NCBI
|
|
2
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Pawel J, Jotte R, Spigel DR, O'Brien
ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts
K, et al: Randomized phase III trial of amrubicin versus topotecan
as second-line treatment for patients with small-cell lung cancer.
J Clin Oncol. 32:4012–4019. 2014. View Article : Google Scholar
|
|
6
|
Desai N, Trieu V, Yao Z, Louie L, Ci S,
Yang A, Tao C, De T, Beals B, Dykes D, et al: Increased antitumor
activity, intratumor paclitaxel concentrations and endothelial cell
transport of cremophor-free, albumin-bound paclitaxel, ABI-007,
compared with cremophor-based paclitaxel. Clin Cancer Res.
15:1317–1324. 2006. View Article : Google Scholar
|
|
7
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small-cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|