Introduction
High-grade astrocytomas are the most difficult
tumors to treat. In 2009, 412 new cases of astrocytomas were
diagnosed in the Czech Republic, which accounts for 3.92 new tumors
per 100,000 people. In total, 57.9% of newly diagnosed cases are
glioblastomas, the most aggressive variant of astrocytomas. Two
types of glioblastoma multiforme (GBM) can be recognised. Secondary
GBMs develop from anaplastic or diffuse astrocytomas. Primary GBMs
do not have any previous developmental phases (1).
Standard treatment includes maximal cytoreductive
surgery followed by concomitant chemotherapy and adjuvant
chemotherapy. Chemoradiotherapy with temozolomide following the
surgery significantly extended overall survival. Median survival of
patients with concomitant chemoradiotherapy was 14.6 vs. 12.1
months without chemotherapy, and 2-year survival was 26 vs. 10%
without chemotherapy (1). Radicality
of resection is an important prognostic factor. According to a 2001
study, the optimal resection denotes >98% of a preoperative
tumor volume. In the case of radical resection the median survival
is 13 vs. 8.8 months in the case of lower radicality (2). Regardless of this comprehensive
treatment, a prognosis of glioblastoma patients remains extremely
poor. The addition of molecular biological examinations to the
complete patient care could improve this unfavorable situation.
These examinations help us to divide patients into individual
prognostic groups and to choose the appropriate therapy, or they
could possibly help us detect formerly unknown factors influencing
the glioblastoma prognosis. From the molecular biological point of
view, the types and numbers of cumulative genetic alterations are
of importance. The important alterations occur in the p53,
PTEN and EGFR genes (3,4). Molecular
biological changes in primary glioblastomas are different from the
molecular biological changes in secondary glioblastomas (3,4).
In the case of secondary glioblastomas developing
from diffuse and anaplastic astrocytomas, detected mutations, such
as the mutation or amplification of the EGFR gene, and the
deletion of the PTEN gene, are found less frequently
compared to the case of primary glioblastomas (3). However, the TP53 deletion is
often detected (3).
Pathophysiological protein kinase and molecular
cascades, particularly p53/MDM2/p14, p16/cyclin-dependent kinase
4/retinoblastoma protein 1 and epidermal growth factor receptor
(EGFR)/phosphatase and tensin homolog (PTEN)/mammalian target of
rapamycin (mTOR)/phosphoinositide 3-kinase/protein kinase B, have
an important role in the pathogenesis and prognosis of GBM
(5,6).
Glioblastomas belong to the group of highly vascular tumors, and
tumor elements produce large quantities of vascular endothelial
growth factor (VEGF) protein. Significantly higher concentrations
of VEGF protein were detected in glioblastoma tissues in comparison
with anaplastic astrocytomas (grade III) and low-grade astrocytomas
(grade II) (7–9). Specific molecular biological changes in
primary glioblastomas are the PTEN gene deletion, loss of
heterozygosity on chromosome 10, the EGFR gene mutation and
amplification, and MDM2 amplification (10). The tumor suppressor PTEN gene
is located on the long arm of chromosome 10 (10q23.3). Its role is
to block the cell cycle in the G1 phase. Malfunction of
the PTEN gene is connected with the progression of low-grade
astrocytomas to more malignant tumors. This mutation detection is
associated with a worse tumor prognosis.
The EGFR gene can be found on the short arm
of chromosome 7 and it is the most frequently amplified oncogene in
primary glioblastomas. EGFR is a membrane receptor for members of
the EGF-family of extracellular ligands. EGFR activation stimulates
intracellular protein-tyrosine kinase activity and
autophosphorylation of several tyrosine (Y) residues in the
C-terminal domain occurs. The activation of signalling cascades of
protein kinases mitogen-activated protein kinase [RAS and B-Raf
proto-oncogene, serine/threonine kinase (BRAF)] (11) leads to DNA synthesis and cell
proliferation. EGFR amplification possibly has a role in diffuse
glioblastoma infiltration into surrounding tissues.
The present case report demonstrates an unusual case
of two independent gliomas in a 44-year-old female patient. A
diffuse astrocytoma and a primary glioblastoma were detected
morphologically and by molecular biological analysis.
Case report
A 44-year-old female patient was examined in the
Department of Neurosurgery (Third Faculty of Medicine, Charles
University in Prague and University Hospital Kralovske Vinohrady in
Prague, Prague, Czech Republic) for headaches that were prominent
for 2 months. Headaches were of an increasing intensity and a
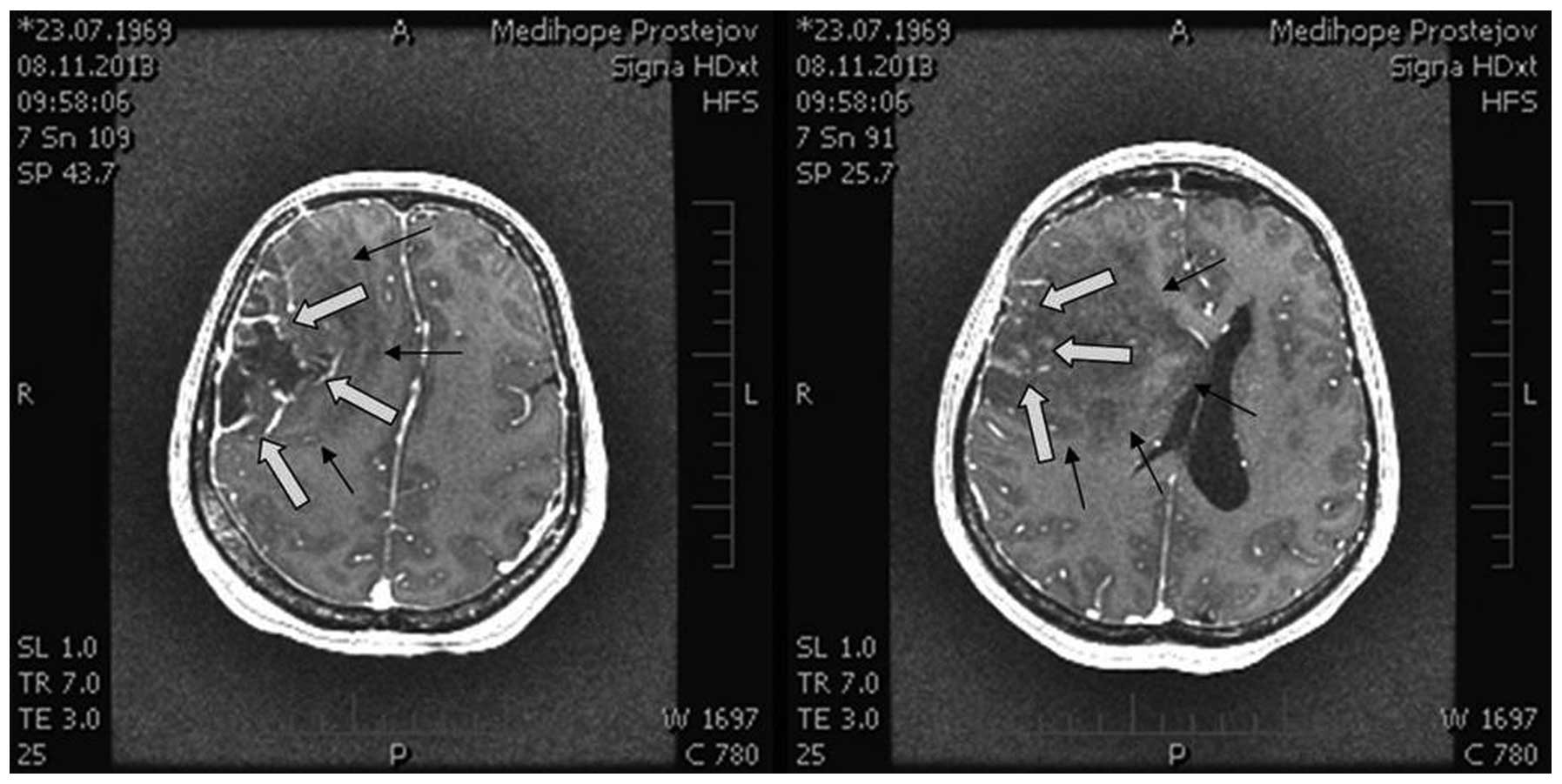
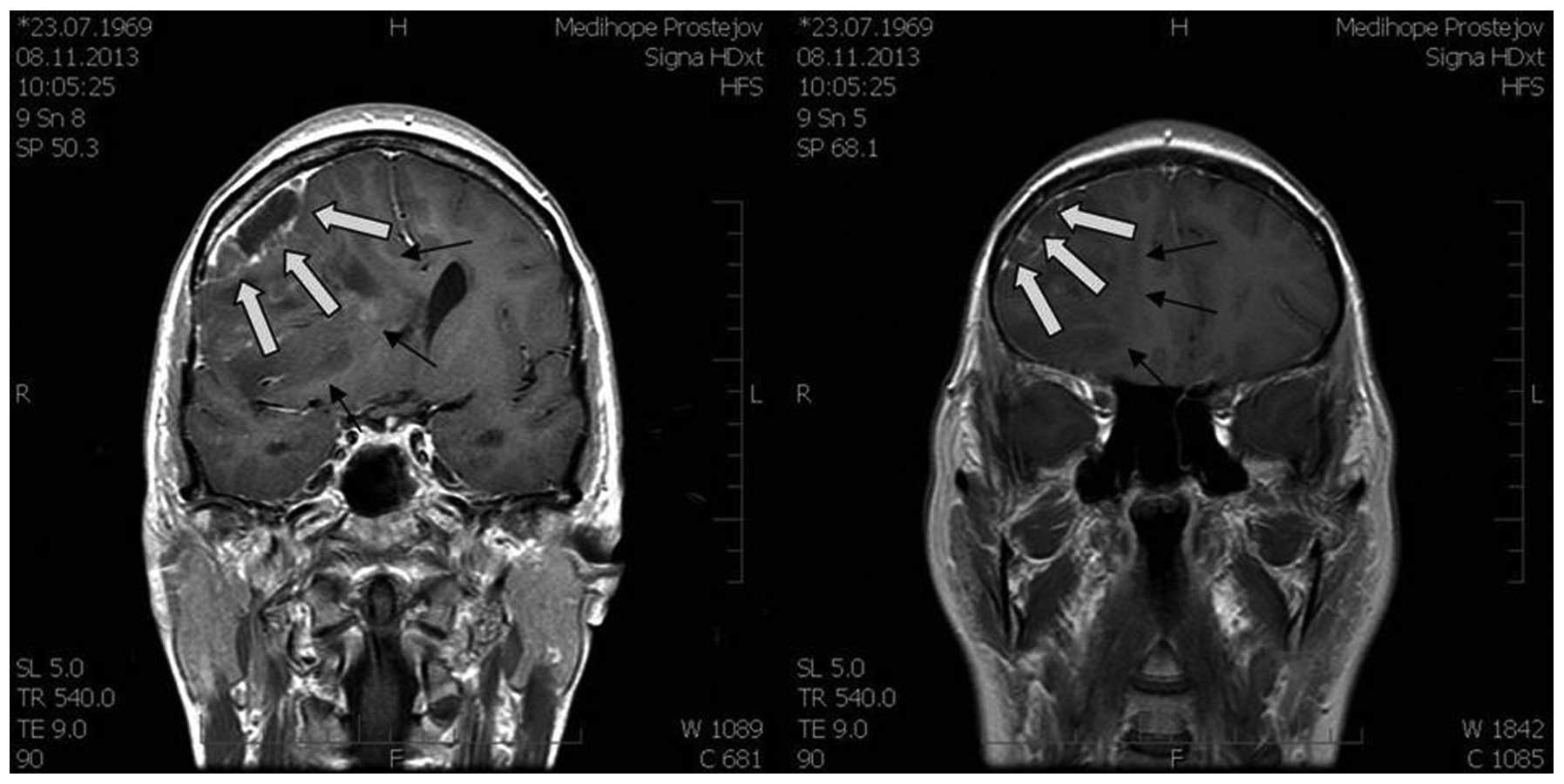
magnetic resonance imaging (MRI) scan (Figs. 1 and 2)
showed a large expansive process in the right frontal lobe
(81×70×76 mm), with the pressure on midline brain structures and
compression of the ventricular system. The MRI scan indicated a
glioma. Neurological examination revealed light left-sided
hemiparesis.
Extirpation of a multicomponent tumor was performed.
On the surface there was a component corresponding with high-grade
gliomas changing quite sharply into a low-grade glioma in the
central section. Tumor extirpation was performed using an operating
microscope, navigation system SonoWand and cavitron ultrasonic
surgical aspirator. The border of a low-grade component was not
visible against surrounding tissues. By contrast, it was well
visible in the sono image and the real-time imaging during the
extirpation of this section of tumor aided with the assessment.
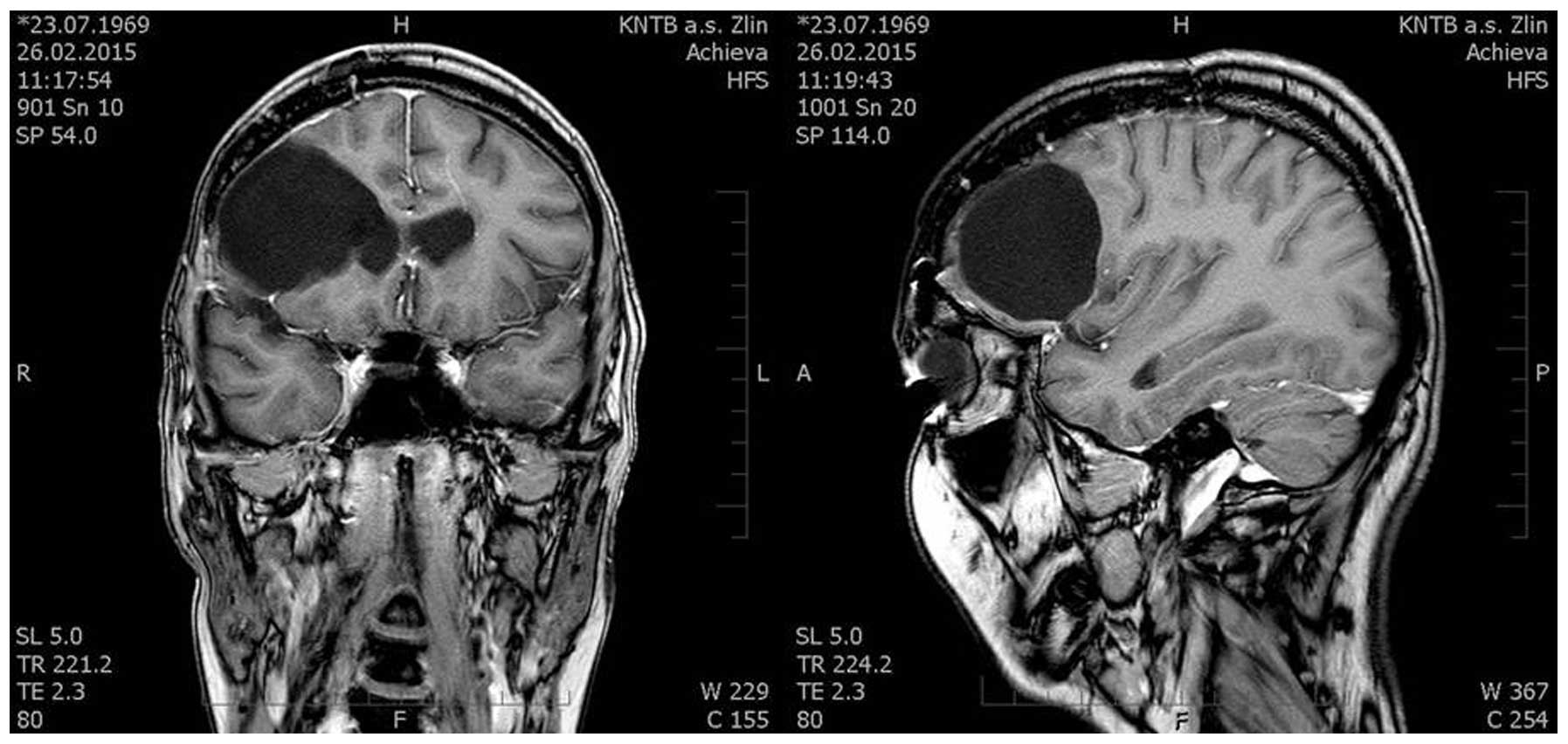
Thus, it was possible to achieve radical resection without
post-contrast enhancement in the follow-up MRI scans. Surgery was
executed without any complications. Left-sided hemiparesis
regressed. The tumor was removed radically, there was no evidence
of disease on the follow-up MRI scans and the patient did not
experience any significant complications (Fig. 3).
Samples were removed from different tumor areas for
histological and molecular biological testing. The tumor component
preoperatively corresponding to a low-grade glioma was
pathologically identified as a diffuse astrocytoma with the
structure of protoplasmic astrocytoma without any signs of necrosis
and microvascular proliferation. In the second tumor component,
preoperatively corresponding to a glioblastoma, high proliferative
activity, necrosis and vascular proliferation were detected and
this section of the tumor was identified as a glioblastoma.
The KRAS, BRAF and EGFR gene
mutations were not detected in the areas of a low-grade tumor.
TP53 deletion was detected cytogenetically, whereas other
markers did not demonstrate any change (Table I). By contrast, the KRAS,
BRAF and EGFR gene mutations were detected in a
glioblastoma. PTEN deletion and EGFR and MDM2
amplifications were detected cytogenetically. These findings
correspond to a primary glioblastoma. Increased mRNA expression for
VEGF was also detected. Immunohistochemical examination
showed increased p53 and mTOR expression. These finding are
also typical for glioblastomas. The examination results are shown
in Table I.
 | Table I.Molecular profile of sample areas
demonstrating the difference between low- and high-grade
glioma. |
Table I.
Molecular profile of sample areas
demonstrating the difference between low- and high-grade
glioma.
| Analysis | High-grade
marker | Low-grade marker |
|---|
| Molecular |
|
|
|
EGFR | Mut exon 18 | WT |
|
KRAS | Mut exon 2 | WT |
|
BRAF | Mut exon 15 | WT |
| FISH |
|
|
|
Her2/neu | No amplification | No amplification |
|
TP53 | Normal | Deletion |
|
PTEN | Deletion | Normal |
|
EGFR | Amplification | No amplification |
|
MDM2 | Amplification | No amplification |
| IHC |
|
|
| p53 | Expression | No expression |
|
mTOR | Expression | No expression |
The patient received adjuvant chemoradiotherapy with
75 mg/m2 temozolomide and radiation therapy 5 times
weekly with 1.8–2.0 Gy/fraction (total dose of 60 Gy). Following
the termination of the treatment, the tiredness reported by the
patient persisted without any other health difficulties. An MRI
scan performed 14 months after the surgery did not show any
evidence of disease.
Discussion
Occurrence of different tumor components in
high-grade brain gliomas is quite common. Areas with high
vascularity and necrosis, and areas showing histological changes
appear. Individual tumor components are usually mixed. In the
previously described tumor, the individual tumor sections
representing different developmental stages were considered as
macroscopically homogeneous and relatively substantially separated.
These findings were confirmed histologically. The question is
whether the section of the tumor corresponding to glioblastoma was
developed from the already present diffuse astrocytoma or if it
developed independently. On the basis of documented analyses and
the accumulation of aberrations, we support the theory of tumor
duplicity. In the high-grade areas, EGFR mutation was
detected (12) together with the
KRAS and BRAF hot-spot gene changes (13). These mutations are not usually
detected when the EGFR gene mutation and amplification is
found. p53 mutation was also detected. p53 and EGFR
mutations are negative prognostic factors for survival. The
detection of PTEN and mTOR gene deletion that was in
the high-grade areas also means a worse prognosis for patients
(14,15).
For the discovery of previously unknown factors that
influence the prognosis of this malignant disease, it is necessary
to study patients with a longer survival and unusual types of
tumors from different angles, including detailed molecular genetic
testing. The case reported in the present study describes an
unusual case of a 44-year old patient with a glioma consisting of
several morphologically different sections from low- to high-grade
and showing the accumulation of genetic alterations. The prognosis
of the patient is determined by the most malignant section of a
tumor. At present, the patient has survived for 15 months after the
surgery without any signs of radiological and clinical disease
progression.
Acknowledgements
The present study was supported by the Ministry of
Education of the Czech Republic (programs PRVOUK-P27/LF1/1 and
PRVOUK-P25/LF1/2).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim B, Myung JK, Seo JH, Park CK, Paek SH,
Kim DG, Jung HW and Park SH: The clinicopathologic values of the
molecules associated with the main pathogenesis of the
glioblastoma. J Neurol Sci. 294:112–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohgaki H and Kleihues P: Genetic
alterations and signaling pathways in the evolution of gliomas.
Cancer Sci. 100:2235–2241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slampa P: Gliomy-soucasná diagnostika a
lecba. Maxdorf. 97–102. 2013.(In Czech).
|
|
7
|
Takano S, Yoshii Y, Kondo S, Suzuki H,
Maruno T, Shirai S and Nose T: Concentration of vascular
endothelial growth factor in the serum and tumor tissue of brain
tumor patients. Cancer Res. 56:2185–2190. 1996.PubMed/NCBI
|
|
8
|
Shim JW, Koh YC, Ahn HK, Park YE, Hwang DY
and Chi JG: Expression of bFGF and VEGF in brain astrocytoma. J
Korean Med Sci. 11:149–157. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oehring RD, Miletic M, Valter MM, Pietsch
T, Neumann J, Fimmers R and Schlegel U: Vascular endothelial growth
factor (VEGF) in astrocytic gliomas-a prognostic factor? J
Neurooncol. 45:117–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toledo F and Wahl GM: MDM2 and MDM4: p53
regulators as target in anticancer therapy. Int J Biochem Cell
Biol. 39:1476–1482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oda K, Matsuoka Y, Funahashi A and Kitano
H: A comprehensive pathway map of epidermal growth factor receptor
signaling. Mol Syst Biol. 1:2005.00102005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verhaak RG, Hoadley KA, Purdom E, Wang V,
Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knobbe CB, Reifenberger J and Reifenberger
G: Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and
BRAF in glioblastomas. Acta Neuropathol. 108:467–470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruano Y, Ribalta T, de Lope AR,
Campos-Martín Y, Fiaño C, Pérez-Magán E, Hernández-Moneo JL,
Mollejo M and Meléndez B: Worse outcome in primary glioblastoma
multiforme with concurrent epidermal growth factor receptor and p53
alteration. Am J Clin Pathol. 131:257–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sesen J, Dahan P, Scotland SJ, Saland E,
Dang VT, Lemarié A, Tyler BM, Brem H, Toulas C, Moyal
Cohen-Jonathan E, et al: Metformin inhibits growth of human
glioblastoma cells and enhances therapeutic response. PLoS One.
10:e01237212015. View Article : Google Scholar : PubMed/NCBI
|