Introduction
Esophageal cancer is one of the most common types of
malignancy in China. Radiotherapy is commonly used concurrently
with chemotherapy as either a definitive or pre-operative treatment
for esophageal carcinoma (1,2). The International Commission on Radiation
Units and Measurements report 62 defined the planning target volume
and described two distinct components: i) The setup margin, which
accounted for uncertainties in the patient position; and ii) the
internal target volume (ITV), which accounted for variations in the
size, shape and position of the tumor (3). Four-dimensional computed tomography
(4D-CT) has improved the accuracy of measurements of the ITV for
tumors that move during respiration, which allows for a more
accurate adjustment of target volumes and a minimization of the
radiation dose delivered to normal tissues, while avoiding a
geographic miss (4,5).
The motion of the centroid in all three dimensions
has been quantitatively evaluated by 4D-CT scanning (6,7). However,
the esophagus is a long tubular organ extending from the neck to
the upper abdomen, which is surrounded by a variety of structures.
Thus, differences in displacement amplitudes of the esophagus
across different segments are likely to be present (8–10).
Esophageal tumors tend to grow and migrate longitudinally along the
esophageal wall. Thus, we hypothesized that differential
displacement of the esophageal tumor may also occur at its proximal
and distal ends. For esophageal cancer, determination of tumor
displacement at the proximal and distal end is particularly
important, as it substantially represents tumor displacement as a
whole. Thus, tumor centroid motion may not be representative of
variations in the shape of the target or movement of its periphery
and ITV margins in the context of the proximal and distal ends of
the tumor, which should be determined separately. However, little
is known regarding these variations in the setting of esophageal
cancer.
Computed tomography has been shown to be relatively
inaccurate in defining the proximal and distal ends of an
esophageal lesion. Using a deformable image registration method,
Zhao et al (6) evaluated
respiratory-induced target motion of esophageal tumors at the
gastro-esophageal junction (GEJ) and found that the deformed
contours commonly featured several ‘islands’ located near the end
when the reference shape of the gross tumor volume (GTV) was drawn
flat with a large contour at the first or the final slice.
However, as deformable image registration methods
were not available at all hospitals, previous studies have
investigated the feasibility of using surgical clips to assess the
volume and localization of the internal GTV based on 4D-CT images.
A previous study has applied this approach for the assessment of
ITV margins with free breathing for external-beam partial breast
irradiation in patients following breast-conserving surgery
(11). Recently,
bronchoscopy-assisted implantation of markers has been used for the
determination of tumor-based setup during image-guided lung cancer
radiotherapy with audiovisual biofeedback (12,13). Metal
objects are rigid, dense and well-defined, and provide a sharp
contrast against the surrounding tissues, which they may be
attached to, rendering them optimal and easily identifiable markers
to track tumor motion.
A previous study revealed that a hemoclip technique
may be safely used to accurately measure the longitudinal GTV of
primary esophageal tumors (14).
The present study prospectively evaluated the
displacement of endoscopically implanted clips at the proximal and
distal ends of the tumor in patients with mid-upper thoracic
esophageal squamous-cell carcinoma and compared differences in
displacement between the upper and lower ends of the tumor in three
dimensions. In this study, tumors located at the upper and middle
thoracic esophagus were assessed, while it has been previously
confirmed that tumors located in the distal esophagus are more
mobile (9,10,15–17).
Therefore, a further exploratory study was performed by placing a
third clip at the lower thoracic esophagus at 2 cm above the
non-neoplastic GEJ to preliminarily determine whether displacement
differences between the upper and lower ends of the tumor may also
occur if the distal end of the tumor is located near the GEJ.
Patients and methods
Study design and patients
A total of 23 patients with esophageal cancer who
were treated at Zhejiang Cancer Hospital (Hangzhou, China) from
September, 2012 to October, 2013 were recruited for this clinical
study. The inclusion criteria were as follows: i) Diagnosed with
mid-upper thoracic esophageal squamous-cell carcinoma with the
upper edge of the tumor located at <30 cm from the incisors; ii)
T1–4, N0-1 and M0-1a stage according to the American Joint
Committee on Cancer staging guidelines (18); iii) a lesion length of ≤8cm; iv)
candidates for radical radiotherapy at the time of CT scanning; v)
cases in which the endoscopic ultrasonography (EUS) probe was able
to pass through the lumen of the esophageal lesions. Only a lesion
length of ≤8 cm was considered as suitable for radical
radiotherapy. Patients with multi-focal lesions were excluded from
the study. Written informed consent was obtained from all subjects
prior to treatment.
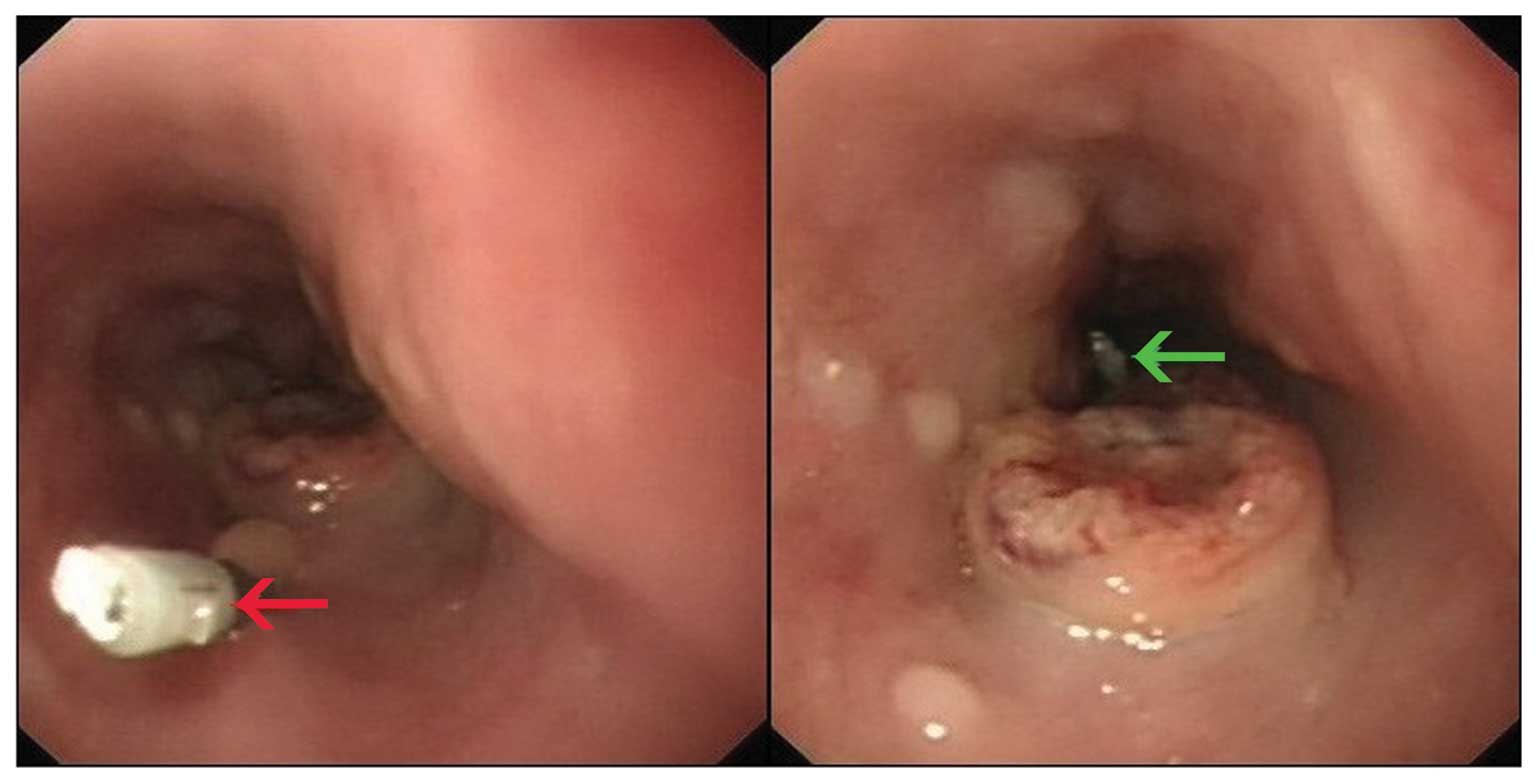
Clip implantation
Prior to 4D-CT simulator examination, the esophageal
tumor was localized using an electronic gastroscope (GIF-Q260;
Olympus, Tokyo, Japan). The clips were then positioned at the
proximal and distal ends of the tumor and, in 16 of the patients, a
third clip was placed at the lower thoracic esophagus 2 cm above
the GEJ (Fig. 1).
4D-CT simulation and image
acquisition
The patients were placed in the supine position with
their arms placed at their sides, with a standard wing board that
was used for immobilization. The Varian Real-time Position
Management (RPM) system (Varian Medical Systems, Palo Alto, CA,
USA) was used to monitor the breathing cycle. All CT images were
obtained from the middle neck to the upper abdomen at a 3-mm slice
thickness upon completion of the standard CT simulation using a
16-slice Brilliance big bore CT scanner (Philips Medical Systems,
Inc., Cleveland, OH, USA) during normal breathing in a resting
state. Following image acquisition, the CT images were
reconstructed for the corresponding respiratory phases according to
the respiratory signal externally acquired by RPM and all CT images
were then automatically sorted into 10 categories that corresponded
to the respiratory phase at which the image was captured using
Varian 4D Integrated Treatment Console Version 13.0 (GE Healthcare,
Little Chalfont, UK).
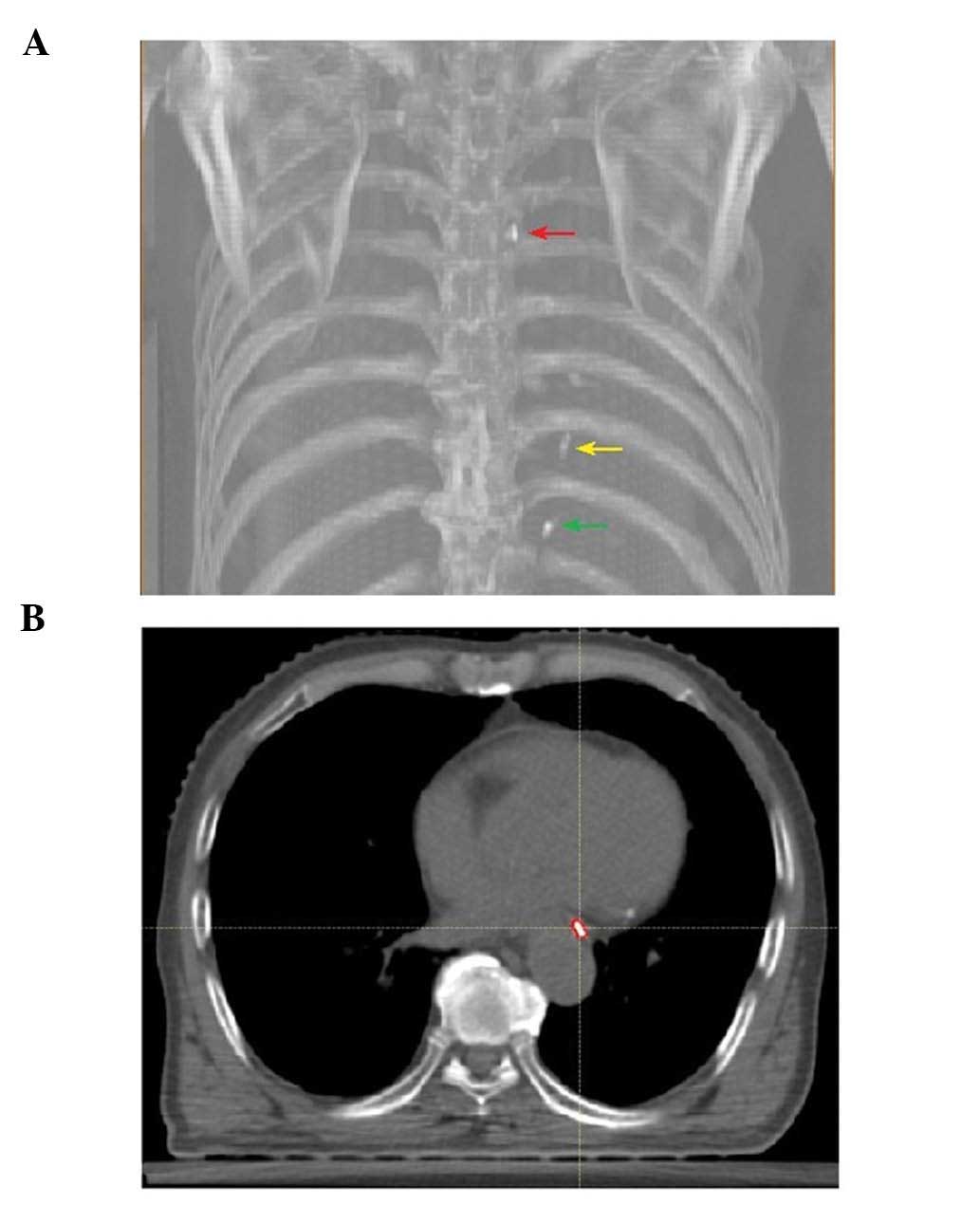
Clip contouring
Upper, lower and cardiac clips were contoured using
a mediastinal window setting for each patient and for all 10
breathing phases of each 4D-CT on a Raystation treatment-planning
workstation (version 4.5.1; Raysearch Laboratories AB, Stockholm,
Sweden). Delineation of clips was performed semi-automatically
(using smart contour software) and then manually edited by a single
radiation oncologist (Fig. 2).
Measurement of clip displacement
To quantify the motion of the clips, the centroid of
each clip was calculated using commercial treatment planning system
software (Raystation version 4.5.1). The centroids of all three
markers were recorded at the 10 respiratory phases. The maximum
differences of the clip centroid position among the 10 breathing
phases in the left-right (LR), superior-inferior (SI) and
anterior-posterior (AP) directions were determined and referred to
as x, y and z, respectively.
Margin expansions for ITV
coverage
The minimum expansion necessary to cover the ITVs of
~95% of the tumors in the LR, SI and AP dimensions was
calculated.
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analyses. For each clip, the
displacement of the clip centroid in three dimensions (x, y and z)
was statistically compared by least-significant differences one-way
analysis of variance. The latter method was also used to compare
the displacements among the upper, lower and cardiac clips in one
direction. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The characteristics of the patients are listed in
Table I. All the patients had upper
and lower clips, and 16 of the patients also had cardiac clips.
None of the patients presented with esophageal bleeding or
perforation. During the CT-based simulation, all clips remained
attached.
 | Table I.Clinical and tumor characteristics of
the 23 patients. |
Table I.
Clinical and tumor characteristics of
the 23 patients.
| Characteristics | n (%) |
|---|
| Age (years) |
|
|
Median | 64 |
| Gender |
|
| Male | 21 (91) |
|
Female | 2 (9) |
| Tumor stage |
|
| T2 | 2 (9) |
| T3 | 19 (82) |
| T4 | 2 (9) |
| Nodal stage |
|
| N0 | 7 (30) |
| N1 | 16 (70) |
| Metastasis stage |
|
| M0 | 15 (65) |
| M1a | 8
(35) |
| Lesion location |
|
| Upper
thoracic | 11 (48) |
| Middle
thoracic | 12 (52) |
| Lesion length
(cm) |
|
| Mean ±
standard deviation | 5.63±0.39 |
|
Range | 3–8 |
Comparison of displacements in three
directions for each clip
For the upper clips, the displacement in LR, SI and
AP direction was 1.04±0.72, 2.54±1.66 and 0.80±0.59 mm,
respectively. For the lower clips, the displacement in LR, SI and
AP direction was 1.26±1.02, 2.61±2.06 and 1.22±0.73 mm,
respectively. For the cardiac clips, the displacement in LR, SI and
AP direction was 2.27±1.06, 5.23±2.43 and 2.07±1.09 mm,
respectively. For each clip, the axial displacement (y) was
significantly greater than the radial displacement (x, z;
P<0.05) and there were no significant differences between the
radial displacements (x, z; P>0.05) (Table II).
 | Table II.Comparison of displacement of the
upper, lower and cardiac clips. |
Table II.
Comparison of displacement of the
upper, lower and cardiac clips.
|
| x (mm) | y (mm) | z (mm) |
|
|---|
|
|
|
|
|
|
|---|
| Clips | Mean | SD | Mean | SD | Mean | SD | P-value |
|---|
| Upper | 1.04 | 0.72 | 2.54 | 1.66 | 0.80 | 0.59 | <0.05 |
| Lower | 1.26 | 1.02 | 2.61 | 2.06 | 1.22 | 0.73 | <0.05 |
| Cardiac | 2.27 | 1.06 | 5.23 | 2.43 | 2.07 | 1.09 | <0.05 |
| P-value | <0.05 | <0.05 | <0.05 |
|
Comparison of displacements among the
three clips
No significant differences in displacement were
found between the upper and lower clips in x, y and z direction.
However, cardiac clips differences in displacement in x, y and z
direction were greater than those of the upper or lower clips
(P<0.05) (Table II).
Margin expansions for ITV
coverage
The minimum expansion required to cover the ITVs of
95% of the tumors in the LR, SI and AP directions was 2.89, 5.00
and 2.36 mm, respectively.
Discussion
In the present study, clips were used as markers to
define the ITV margins of the proximal and distal ends of
esophageal tumors that occurred at the mid-upper thoracic
esophagus. The results revealed that the axial displacement was
significantly greater than the radial displacement and asymmetric
margins to cover ~95% of RL, SI and AP motion were 2.89, 5.00 and
2.36 mm, respectively. The proximal and distal ends of the tumors
had a similar magnitude of displacement in the RL, SI and AP
direction. Thus, it is not recommended to set differential ITV
margins based on different parts of the tumor for mid-upper
esophageal cancer.
Several studies have previously reported directional
displacement differences in primary esophageal cancer (6,10,15). Zhao et al (6) studied tumors located at the GEJ using
respiratory-induced target motions by 4D-CT and recommended margins
of 1.0 cm (left), 0.8 cm (right), 1.1 cm (anterior), 0.6 cm
(posterior), 1.0 cm (superior) and 1.6 cm (inferior). Hashimoto
et al (10) also found that
the mean range of motion of fiducial markers inserted into the
esophageal wall was 3.5±1.8 mm, 8.3±3.8 and 4.0±2.6 mm,
respectively, for the medio-lateral, cranio-caudal and AP
directions. Furthermore, a study by Patel et al (15) using time-resolved 4D-CT revealed that
the peak-to-peak displacements of all primary tumors in the SI, AP
and LR dimensions were 0.80±0.45, 0.28±0.20 and 0.22±0.23 cm,
respectively. The results of the present study were concordant with
those determined in the abovementioned studies, while the magnitude
of motion in the SI direction (5.00 mm) determined by the present
study was smaller. This was partly due to the fact that the lesions
of all patients of the present study were located at the mid-upper
thoracic esophagus, which have been reported to have a reduced
motion compared to those at the distal esophagus (9,10).
To the best of our knowledge, the present study was
the first to evaluate whether displacement differences exist
between the upper and lower ends of an esophageal tumor that is
located at the upper-middle thoracic esophagus. Patients with this
type of esophageal cancer are considered to be prime candidates for
definitive radiotherapy, highlighting the importance of the
findings of the present study. Our results demonstrated that no
significant differences in displacement were found between the
upper and lower clips in the LR, SI and AP directions and, thus,
differential ITV margins based on different parts of the tumor are
not recommended. Previous studies have investigated the
displacement of tumors in various parts of the esophagus with
conflicting results (9,10,15–17). For
example, in a study by Lorchel et al (16), 8 patients that presented with
esophageal malignancies underwent two conventional spiral CT scans
during breath-hold procedure under spirometric control, and no
association of tumor motion with anatomical location was
observed.
Other studies have reported that tumors in the
distal esophagus had higher mobility compared with those in the
middle and upper esophagus (9,10,15,17). The
aim of the present study was to investigate the displacement
differences between the superior and inferior ends of tumors
located in the upper-middle thoracic esophagus in three directions.
However, the results were not in agreement with two previous
studies reporting that intrafractional esophageal displacement
varied depending on the location in the normal esophagus (8,9). This
finding allows for the hypothesis that the neoplastic esophagus
moves in different ways from the healthy esophagus.
However, further analysis using clips at the lower
thoracic esophagus near the GEJ revealed a significantly larger
motion in three directions. This suggested that the ITV margins of
the superior and inferior ends of esophageal tumors should be
determined separately in patients in wom the lower edge of the
lesion is located near the GEJ. A study by Hashimoto et al
(10) evaluating the feasibility of
real-time monitoring of fiducial markers in the digestive tract
followed by analysis of the motion of the healthy esophagus
concluded that respiration and heartbeat (particularly) were the
main causes of esophageal motion.
Furthermore, the esophagus near the GEJ may be
subject to considerable respiratory diaphragmatic motion, becoming
more mobile. However, the observations of the present study should
be interpreted with caution, as the data of this exploratory study
were derived from the displacement of clips attached to the normal
distal esophagus, which may be mobile in ways that are different
from the esophagus bearing tumors. Therefore, a further prospective
study is required to assess the displacement of clips attached to
tumors whose distal end is located in proximity to the GEJ.
Furthermore, the accuracy of the calculation of the marker
centroids was limited, considering that the use of 3-mm CT slices
affected the accuracy of the position of the markers by 1.0 cm.
These errors may be reduced by acquiring CT images with a lower
axial slice thickness or by volumetric acquisition (17,19).
In conclusion, to the best of our knowledge, the
present study was the first to use clips and 4D-CT imaging to
determine the ITV margins of upper-middle esophageal cancer. The
minimum expansion of ITV margins required to cover 95% of the RL,
SI and AP motion were calculated to be 2.89, 5.00 and 2.36 mm,
respectively. For the upper and lower clips, axial displacement (y)
was greater than radial displacement (x, z; P<0.05), indicating
that axial and radial ITV margins should be determined separately.
It was also revealed that LR, SI and AP displacement of clips
attached to the normal GEJ was greater than that of upper or lower
clips (P<0.05); therefore, further study is required on patients
in whom the distal tumor end is located in proximity to the
GEJ.
References
|
1
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Hagen P, Hulshof MC, van Lanschot JJ,
et al: Preoperative chemoradiotherapy for esophageal or junctional
cancer. N Engl J Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
International Commission on Radiation
Units and Measurements. ICRU report 62: Prescribing, recording and
reporting photon beam therapy (supplement to ICRU report 50)
(Washington, DC). ICRU. 1999.
|
|
4
|
Admiraal MA, Schuring D and Hurkmans CW:
Dose calculations accounting for breathing motion in stereotactic
lung radiotherapy based on 4D-CT and the internal target volume.
Radiother Oncol. 86:55–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yakoumakis N, Winey B, Killoran J, Mayo C,
Niedermayr T, Panayiotakis G, Lingos T and Court L: Using
four-dimensional computed tomography images to optimize the
internal target volume when using volume-modulated arc therapy to
treat moving targets. J Appl Clin Med Phys. 13:38502012.PubMed/NCBI
|
|
6
|
Zhao KL, Liao Z, Bucci MK, Komaki R, Cox
JD, Yu ZH, Zhang L, Mohan R and Dong L: Evaluation of
respiratory-induced target motion for esophageal tumors at the
gastroesophageal junction. Radiother Oncol. 84:283–289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yaremko BP, Guerrero TM, McAleer MF, Bucci
MK, Noyola-Martinez J, Nguyen LT, Balter PA, Guerra R, Komaki R and
Liao Z: Determination of respiratory motion for distal esophagus
cancer using four-dimensional computed tomography. Int J Radiat
Oncol Biol Phys. 70:145–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan CC, Kashani R, Hayman JA, et al:
Intra- and inter-fraction esophagus motion in 3D-conformal
radiotherapy: Implications for ICRU 62 definitions of internal
target volume and planning organ at risk volume. Int J Radiat Oncol
Biol Phys. 60(Suppl): S580–S581. 2004. View Article : Google Scholar
|
|
9
|
Dieleman EM, Senan S, Vincent A,
Lagerwaard FJ, Slotman BJ and van Sörnsen de Koste JR:
Four-dimensional computed tomographic analysis of esophageal
mobility during normal respiration. Int J Radiat Oncol Biol Phys.
67:775–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimoto T, Shirato H, Kato M, Yamazaki
K, Kurauchi N, Morikawa T, Shimizu S, Ahn YC, Akine Y and Miyasaka
K: Real-time monitoring of a digestive tract marker to reduce
adverse effects of moving organs at risk (OAR) in radiotherapy for
thoracic and abdominal tumors. Int J Radiat Oncol Biol Phys.
61:1559–1564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Li J, Wang W, Wang S, Wang J, Ma
Z, Shao Q and Xu M: A comparative study on the volume and
localization of the internal gross target volume defined using the
seroma and surgical clips based on 4DCT scan for external-beam
partial breast irradiation after breast conserving surgery. Radiat
Oncol. 9:762014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roman NO, Shepherd W, Mukhopadhyay N, Hugo
GD and Weiss E: Interfractional positional variability of fiducial
markers and primary tumors in locally advanced non-small-cell lung
cancer during audiovisual biofeedback radiotherapy. Int J Radiat
Oncol Biol Phys. 83:1566–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueki N, Matsuo Y, Nakamura M, Mukumoto N,
Iizuka Y, Miyabe Y, Sawada A, Mizowaki T, Kokubo M and Hiraoka M:
Intra- and interfractional variations in geometric arrangement
between lung tumours and implanted markers. Radiother Oncol.
110:523–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu GQ, Du XH, Yu JP, Zheng YD, Luo HJ, Xu
YP, Chen JX, Sun XJ, Ji YL and Tao YL: The value of endoscopic
ultrasonography in defining longitudinal gross target volumes for
esophageal squamous carcinoma. Surg Laparosc Endosc Percutan Tech.
22:424–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel AA, Wolfgang JA, Niemierko A, Hong
TS, Yock T and Choi NC: Implications of respiratory motion as
measured by four-dimensional computed tomography for radiation
treatment planning of esophageal cancer. Int J Radiat Oncol Biol
Phys. 74:290–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lorchel F, Dumas JL, Noël A, Wolf D,
Bosset JF and Aletti P: Esophageal cancer: Determination of
internal target volume for conformal radiotherapy. Radiother Oncol.
80:327–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamashita H, Kida S, Sakumi A, Haga A, Ito
S, Onoe T, Okuma K, Ino K, Akahane M, Ohtomo K and Nakagawa K:
Four-dimensional measurement of the displacement of internal
fiducial markers during 320-multislice computed tomography scanning
of thoracic esophageal cancer. Int J Radiat Oncol Biol Phys.
79:588–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greene FL, Page DL, Fleming ID, et al:
AJCC Cancer Staging Manual (6th). Springer-Verlag. New York: 2002.
View Article : Google Scholar
|
|
19
|
Yamashita H, Okuma K, Tada K, Shiraishi K,
Takahashi W, Shibata-Mobayashi S, Sakumi A, Saotome N, Haga A, Onoe
T, et al: Four-dimensional measurement of the displacement of
internal fiducial and skin markers during 320-multislice computed
tomography scanning of breast cancer. Int J Radiat Oncol Biol Phys.
84:331–335. 2012. View Article : Google Scholar : PubMed/NCBI
|