Introduction
Colorectal cancer (CRC) is frequently encountered in
clinical practice (1). The majority
of CRCs develop slowly from colon polyps, due to the
adenoma-carcinoma sequence (2). To
improve the prognosis of patients with CRC, prompt and accurate
diagnosis is crucial. CRC is screened with fecal occult blood
testing and diagnosed with colonoscopy (3). However, fecal occult blood testing is
not entirely reliable, although no other modalities surpass this
test regarding practicality and affordability (4). Colonoscopy is the gold standard of
diagnostic methods for CRC. However, colonoscopy is not available
to all patients, as not many clinicians are adequately skilled to
perform this procedure (5).
Abdominal ultrasound (US) is useful for the safe and
easy diagnosis of CRC patients (6–9). CRC is
occasionally diagnosed with abdominal US during investigation of
patients with abdominal symptoms or anemia (9). A thickened colonic wall is a clue to the
diagnosis of CRC (10). The threshold
value for the diagnosis of CRC, however, has not yet been
determined. Stratification and contour illustrated with abdominal
US are associated with the depth of invasion, either to the
subserosa (SS) or the subserosa (SE) (11). If stratification and contour are
associated with the morphology of CRC, such as wall thickness (W)
or mass (M), morphology may designate the depth of invasion
(11).
We retrospectively investigated patient records to
determine the characteristics of CRC diagnosed with screening
abdominal US. Blood test variables were also analyzed to assess
patient backgrounds.
Patients and methods
Ethics statement
This study was approved by the National Hospital
Organization Shimoshizu Hospital Ethics Committee. This was not
considered to be a clinical trial, as the procedures were performed
as a part of routine clinical practice. Written informed consent
was obtained from the patients to perform colonoscpy. Informed
consent was obtained to perform abdominal US, but written forms
were waived. Written informed consent for inclusion in the study
was waived, as patient records were anonymized and retrospectively
analyzed.
Patients
The medical records of patients who were treated at
the National Hospital Organization Shimoshizu Hospital from March,
2010 to January, 2015 were retrospectively analyzed. Enrolled
patients were required to meet the following inclusion criteria:
Subjected to abdominal US prior to colonoscopy, computed tomography
(CT), or magnetic resonance imaging; underwent surgery at the
National Hospital Organization Shimoshisu Hospital; and diagnosis
pathologically confirmed. The patients underwent abdominal US for
anemia, abdominal pain and bowel obstruction. Certain patients were
subjected to abdominal US for screening. Following diagnosis of CRC
with abdominal US, colonoscopy was performed in all the patients.
The exclusion criteria were as follows: Subjected to abdominal US
after the diagnosis of CRC with colonoscopy; subjected to abdominal
US with the suspicion of CRC with CT or magnetic resonance imaging;
and not subjected to surgery. The enrolled patients were restricted
to those whose surgical specimens were available to investigate the
depth of invasion. The enrolled patients included 5 men (aged
74.0±0.8 years) and 10 women (aged 73.0±12.0 years).
Abdominal US
Abdominal US was performed by Senior Fellows of the
Japan Society of Ultrasonics in Medicine (M.T. and F.S) using the
SSA-700A diagnostic US system (Toshiba Medical Systems Corporation,
Ohtawara, Japan) with a 3.75-MHz curved-array probe (PVT-375BT;
Toshiba Medical Systems) or an 8.0-MHz linear-array probe
(PLT-805AT; Toshiba Medical Systems) in the US unit. The small and
large intestines were scanned following routine abdominal US when
intestinal diseases, such as ileus, were suspected, or when the
patients had anemia.
Criteria for the diagnosis of CRC
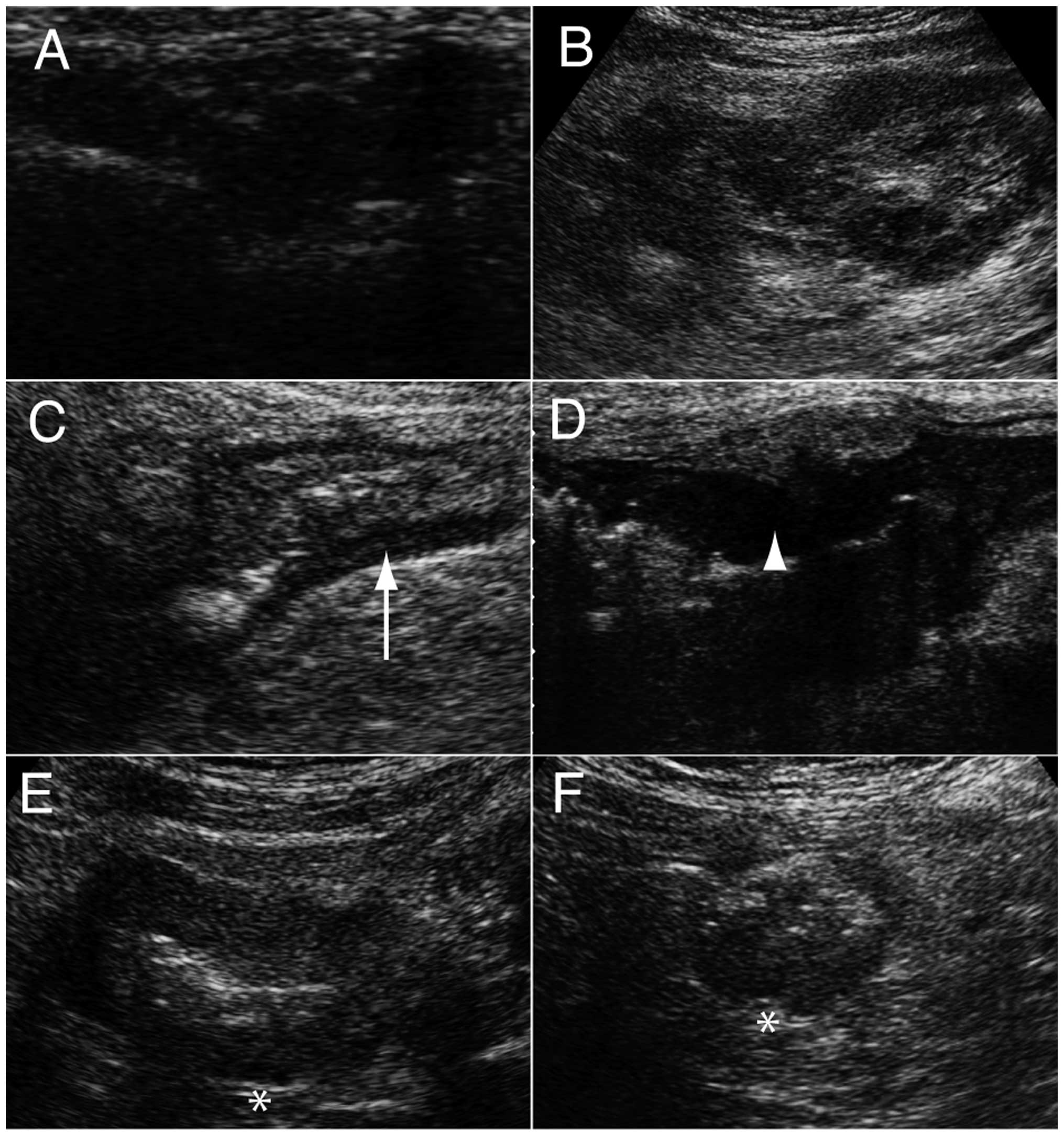
The diagnostic criterion for CRC was localized
irregular wall thickening (Fig. 1A)
or a hypoechoic mass with a hyperechoic mass (pseudokidney sign;
Fig. 1B) (10). The former is a common finding in
patients with CRC (12), while the
latter represents tumor tissue and air in the residual lumen
(13).
Wall thickness, shape, stratification
and contour of CRC
Wall thickness was measured with abdominal US
between the mucosa and serosa borders. Wall thickness was analyzed
to differentiate between CRC and the surrounding normal colonic
wall. The US findings were evaluated in terms of shape,
stratification and contour. Shape was divided into wall thickening
(W; Fig. 1A) and mass (M; Fig. 1B). Stratification was observed due to
the different layers of the colonic wall (12) and patients were divided into two
groups, namely preserved (Fig. 1C) or
lost stratification (Fig. 1D).
Irregular contour is considered to be an US characteristic of CRC
(10). A proportion of the patients
had a smooth contour (Fig. 1E), while
the majority exhibited irregular contour (Fig. 1F).
Pathological analysis
The depth of invasion was determined by two
pathologists (K.F. and T.K). The analyzed specimens were obtained
via surgical resection. Patients referred to other hospitals for
pathological analysis and those treated conservatively were
excluded from the analysis.
Blood test variables
The blood test variables analyzed were white blood
cell count, hemoglobin (Hb), C-reactive protein (CRP),
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA
19-9).
Statistical analysis
The mean wall thickness was compared between CRC and
the surrounding normal colonic wall with one-way analysis of
variance. The Chi-square test was applied to analyze the
correlation between the shape of CRC (W or M) and stratification or
contour. The Chi-squared test was also applied to analyze the
correlation between depth of invasion and the shape of CRC (W or
M), stratification, or contour. The threshold value of wall
thickness to diagnose CRC was investigated with receiver operating
characteristic (ROC) curve analysis. A P-value of <0.05
indicated statistically significant differences. JMP 10.0.2
software (SAS Institute, Cary, NC) was used for all statistical
analyses.
Results
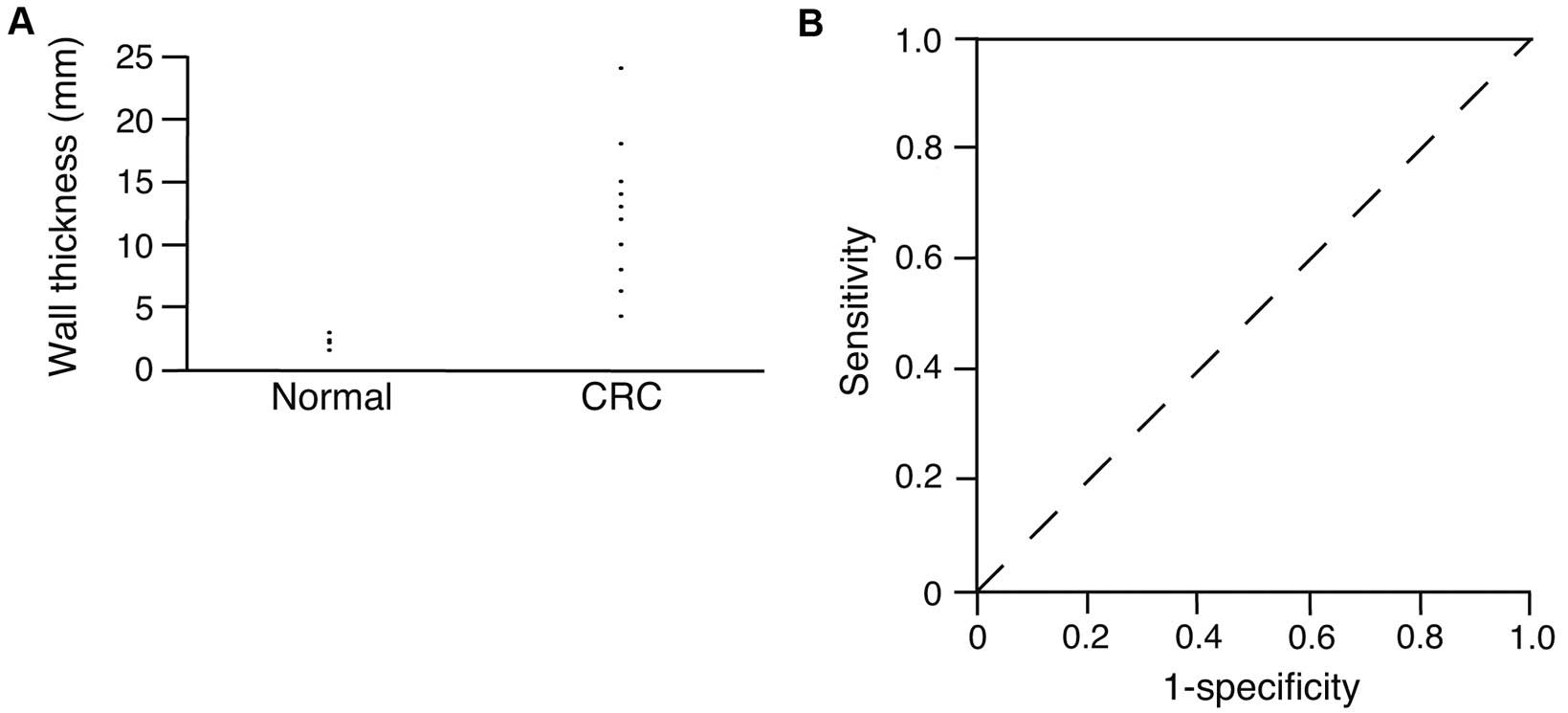
Comparison of wall thickness between
CRC and normal colon
The wall thickness in CRC and in the surrounding
normal colonic wall was measured and plotted in Fig. 2A. The average wall thickness was
2.8±0.4 mm in the surrounding normal tissue and 12.7±5.2 mm in CRC.
The wall was significantly thicker in CRC compared with the normal
colonic wall (P<0.0001). The thickness of normal colonic wall
was <3.0 mm, while it was >4.3 mm in CRC. As shown in
Fig. 2A, there may be a threshold
value for the diagnosis of CRC using wall thickness. ROC curve
analysis was performed to investigate the threshold value for the
diagnosis of CRC using abdominal US. The calculated threshold value
was 4.3 mm. The sensitivity and specificity at this value were both
100%.
Correlation of stratification and
contour with shape in CRC
To determine whether there is an association between
the shape of CRC and stratification or contour, Chi-square test was
performed (Table I). Stratification
was preserved in W, while it was lost in M (P=0.0196). The
correlation between shape and contour was not significant
(P=0.4356).
 | Table I.Correlation of the stratification or
contour with the shape of colorectal cancers. |
Table I.
Correlation of the stratification or
contour with the shape of colorectal cancers.
|
| Stratification
(P=0.0196) | Contour
(P=0.4356) |
|---|
|
|
|
|
|---|
| Shape | Preserved | Lost | Smooth | Irregular | Total |
|---|
| W | 3 | 4 | 2 | 5 | 7 |
| M | 0 | 8 | 1 | 7 | 8 |
| Total | 3 | 12 | 2 | 12 | 15 |
Correlation of depth of invasion with
shape, stratification and contour in CRC
To analyze the association between the depth of
invasion and the shape, stratification, or contour, Chi-square test
was performed (Table II). No
significant correlation was observed between any of the
variables.
 | Table II.Correlation of depth of invasion of
colorectal cancer with shape, stratification and contour. |
Table II.
Correlation of depth of invasion of
colorectal cancer with shape, stratification and contour.
|
| W or M
(P=0.1292) | Stratification
(P=0.1225) | Contour
(P=0.4686) |
|---|
|
|
|
|
|
|---|
| Depth of
invasion | W | M | Preserved | Lost | Smooth | Irregular | Total |
|---|
| MP | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| SS | 6 | 6 | 2 | 10 | 3 | 9 | 12 |
| SE | 0 | 2 | 0 | 2 | 0 | 2 | 2 |
| Total | 7 | 8 | 3 | 12 | 3 | 12 | 15 |
Laboratory findings in colorectal
cancer patients
To assess the background of patients diagnosed with
CRC using abdominal US, blood test variables were analyzed
(Table III). The Hb level was below
the normal range, whereas the CRP, CEA and CA 19-9 levels were
above the normal range.
 | Table III.Laboratory findings in colorectal
cancer patients. |
Table III.
Laboratory findings in colorectal
cancer patients.
| Findings | Range | Mean ± SD | Normal |
|---|
| WBC
(×103/µl) | 3.1–19.0 |
8.0±4.5 | 3.5–8.5 |
| Hb (g/dl) | 3.9–14.7 |
10.8±5.3 | 11.5–15.0 |
| CRP (mg/dl) | 0.2–14.1 |
4.2±5.3 | 0–0.3 |
| CEA (ng/ml) | 1.5–44.2 |
18.9±17.9 | 0–5 |
| CA19–9 (U/ml) | 5.3–595 |
63.2±168 | 0–37 |
Discussion
The threshold value of colonic wall thickness on
abdominal US may be useful for the diagnosis of CRC. The upper
limit of the normal colonic wall is 3 mm on CT (14). Stermer et al performed
colonoscopy in patients who had a wall thickened to >3 mm
(15). Of the 46 patients, 30 had a
wall thicker than 3 mm, but showed no abnormalities, suggesting
that false-positive results may be found in patients with walls
thicker than 3 mm; thus, the threshold value may be >3 mm. In
our study, the thickness of normal colonic wall was <3 mm. Our
data were consistent with previous results (15). A threshold value for colonic wall
thickness has not been determined for the diagnosis of CRC. Our
data clearly demonstrated a threshold value of 4.3 mm. The wall
thickness in CRC has been reported to be 14 mm at the time of
diagnosis with CT (16), suggesting
that the threshold value of wall thickness for the diagnosis of CRC
may be lower with abdominal US. This hypothesis may be supported by
the fact that abdominal US provides more detailed findings compared
with CT (11).
Loss of stratification is observed in 85% of
patients with CRC (12). In our
study, stratification was lost in patients with the M type of CRC.
CRC is more advanced in the M type compared with the W type. Our
data are supported by the fact that loss of stratification
indicates CRC cell invasion (11).
Regarding rectal cancer, endorectal US is suitable for the
evaluation of the extent and staging of rectal cancer (17,18).
However, endorectal US is not suitable for screening, in contrast
to abdominal US. Moreover, our data clearly indicated that
abdominal US was useful for the evaluation of the extent of
CRC.
Our data demonstrated that the Hb level was lower
and CRP was higher compared with the normal values in patients with
CRC. It has been demonstrated that CRC is associated with bleeding
and inflammation (19). An elevated
CRP level indicates that CRC is advanced and the prognosis is poor
(20). Lower Hb level is associated
with Dukes stages B and C, rather than with stage A (21). CEA and CA 19-9 are known markers of
CRC (22). Our results demonstrated
that the CEA and CA 19-9 levels were higher compared with the
normal values. CEA correlates with disease-free survival after
surgery for CRC (23). These results
and previous reports suggest that CRC diagnosed with abdominal US
is advanced.
The major limitation of our study was the small
number of patients, as the enrolled patients were restricted to
those diagnosed with CRC using abdominal US.
In conclusion, the threshold value of colonic wall
thickness was 4.3 mm for the diagnosis of CRC with abdominal US.
CRC was advanced at diagnosis, with higher CRP, CEA and CA 19-9
levels, and lower Hb levels.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Sohaily S, Biankin A, Leong R,
Kohonen-Corish M and Warusavitarne J: Molecular pathways in
colorectal cancer. J Gastroenterol Hepatol. 27:1423–1431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stracci F, Zorzi M and Grazzini G:
Colorectal cancer screening: tests, strategies, and perspectives.
Front Public Health. 2:2102014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benton SC, Seaman HE and Halloran SP:
Faecal occult blood testing for colorectal cancer screening: The
past or the future. Curr Gastroenterol Rep. 17:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wallace MB and Kiesslich R: Advances in
endoscopic imaging of colorectal neoplasia. Gastroenterology.
138:2140–2150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puylaert JB, van der Zant FM and Rijke AM:
Sonography and the acute abdomen: Practical considerations. AJR Am
J Roentgenol. 168:179–186. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laméris W, van Randen A, Dijkgraaf MG,
Bossuyt PM, Stoker J and Boermeester MA: Optimization of diagnostic
imaging use in patients with acute abdominal pain (OPTIMA): Design
and rationale. BMC Emerg Med. 7:92007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhillon S, Halligan S, Goh V, Matravers P,
Chambers A and Remedios D: The therapeutic impact of abdominal
ultrasound in patients with acute abdominal symptoms. Clin Radiol.
57:268–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Ultrasonography for
leukocytosis or elevated C-reactive protein.
Hepatogastroenterology. 58:1156–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shirahama M, Koga T, Ishibashi H, Uchida S
and Ohta Y: Sonographic features of colon carcinoma seen with
high-frequency transabdominal ultrasound. J Clin Ultrasound.
22:359–365. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomizawa M, Shinozaki F, Hasegawa R, Fugo
K, Shirai Y, Ichiki N, Sugiyama T, Yamamoto S, Sueishi M and
Yoshida T: Screening ultrasonography is useful for the diagnosis of
gastric and colorectal cancer. Hepatogastroenterology. 60:517–521.
2013.PubMed/NCBI
|
|
12
|
Truong M, Atri M, Bret PM, Reinhold C,
Kintzen G, Thibodeau M, Aldis AE and Chang Y: Sonographic
appearance of benign and malignant conditions of the colon. AJR Am
J Roentgenol. 170:1451–1455. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Malley ME and Wilson SR: US of
gastrointestinal tract abnormalities with CT correlation.
Radiographics. 23:59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fisher JK: Normal colon wall thickness on
CT. Radiology. 145:415–418. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stermer E, Lavy A, Rainis T, Goldstein O,
Keren D and Zeina AR: Incidental colorectal computed tomography
abnormalities: Would you send every patient for a colonoscopy? Can
J Gastroenterol. 22:758–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi SJ, Kim HS, Ahn SJ, Jeong YM and Choi
HY: Evaluation of the growth pattern of carcinoma of colon and
rectum by MDCT. Acta Radiol. 54:487–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heo SH, Kim JW, Shin SS, Jeong YY and Kang
HK: Multimodal imaging evaluation in staging of rectal cancer.
World J Gastroenterol. 20:4244–4255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu D, Ju HX, Qian CW and Jiang F: The
value of TRUS in the staging of rectal carcinoma before and after
radiotherapy and comparison with the staging postoperative
pathology. Clin Radiol. 69:481–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomizawa M, Shinozaki F, Hasegawa R,
Togawa A, Shirai Y, Ichiki N, Motoyoshi Y, Sugiyama T, Yamamoto S
and Sueishi M: Reduced hemoglobin and increased C-reactive protein
are associated with upper gastrointestinal bleeding. World J
Gastroenterol. 20:1311–1317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sugano K, Ikeya T, Kimura K, Amano R, Kubo N, Tanaka H, et al:
Elevated preoperative serum C-reactive protein levels are
associated with poor survival in patients with colorectal cancer.
Hepatogastroenterology. 61:2236–2240. 2014.PubMed/NCBI
|
|
21
|
Khanbhai M, Shah M, Cantanhede G, Ilyas S
and Richards T: The problem of anaemia in patients with colorectal
cancer. ISRN Hematol. 2014:5479142014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stiksma J, Grootendorst DC and van der
Linden PW: CA 19-9 as a marker in addition to CEA to monitor
colorectal cancer. Clin Colorectal Cancer. 13:239–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Destri G, Rubino AS, Latino R, Giannone
F, Lanteri R, Scilletta B and Di Cataldo A: Preoperative
carcinoembryonic antigen and prognosis of colorectal cancer. An
independent prognostic factor still reliable. Int Surg.
100:617–625. 2015. View Article : Google Scholar : PubMed/NCBI
|