Introduction
Gastric cancer is a relatively common type of
cancer, and is the second leading cause of cancer mortality in the
world. In excess of 700,000 deaths of patients with gastric cancer,
and almost 1,000,000 new gastric cancer cases, occur globally each
year (1). Gastric cancer has an
extremely poor prognosis. The 5-year survival rates for gastric
cancer are low in most countries, at <30% (2). Surgical resection is the only
potentially curative therapy for gastric cancer. However, only a
minority of patients with gastric cancer are suitable for surgical
treatment, predominantly due to the high proportion of advanced
tumors at the time of presentation (2). Thus, radical operation remains the most
important therapeutic means for patients with gastric cancer to
achieve long-term survival. The classification of the
lymphadenectomy of gastric cancer is a vital link for improving
treatment. The latest lymph node (N) staging of gastric cancer is
determined by calculating the number of lymph nodes (3,4), which
requires lymph node harvesting to accurately determine the staging
for patients with gastric cancer. Thus, the anatomical structure of
complex tissues of lymph node resection, such as No. 12a lymph
nodes, is receiving increased attention.
The 7th edition guidelines of the American Joint
Committee on Cancer (AJCC) for gastric cancer have been rigorously
debated (5,6). One of the changes in these guidelines is
that No. 12a lymph nodes are no longer assigned to D2
lymphadenectomy [numerous surgeons agree that D2 lymphadenectomy is
a standard surgical procedure for gastric cancer (7,8)], and No.
12a metastasis has been reclassified accordingly as distant
metastasis.
However, the 3rd edition of the Japanese treatment
guidelines and the 6th edition of the AJCC guidelines for gastric
cancer concur that No. 12a lymph node metastasis is a type of
regional metastasis from a primary gastric cancer, and should be
dissected during D2 lymphadenectomy to improve patient outcome
(9,10). The difference between the guidelines
may cause confusion in surgeons.
The scope definition of No. 12a group lymph nodes is
undefinable according to the AJCC guidelines, Japanese treatment
guidelines and the National Comprehensive Cancer Network. None of
these guidelines are able to describe the scope definition of No.
12a group lymph nodes for gastric cancer. These guidelines
completely reference the guidelines for biliary carcinoma
processing, which indicate that No. 12a group lymph nodes may be
located along the proper hepatic artery (11).
The aim of the present study was to provide a clear
and practical scope definition of No. 12a group lymph nodes
according to our clinical experiences and practices, and to
evaluate the clinical importance and survival outcomes of patients
with gastric cancer with No. 12a lymph node metastasis following D2
lymphadenectomy.
Materials and methods
The present study was approved by the Institutional
Review Board of the first Hospital Affiliated to Fujian Medical
University, and informed consent was obtained according to
institutional regulations. Written informed consent for further
clinical research was obtained from participants for their clinical
records.
Clinical data collection
Data obtained from patients with gastric cancer who
received gastrectomy plus D2 or greater lymphadenectomy between
January 2000 and December 2009 at the first Hospital Affiliated to
Fujian Medical University were retrospectively analyzed. The
inclusion criteria were as follows: i) advanced gastric cancer; ii)
carcinoma (including adenocarcinoma, mucinous or signet ring
adenocarcinoma) confirmed by histopathology; iii) D2 or greater
lymphadenectomy; iv) patients did not receive neoadjuvant
chemotherapy or chemoradiotherapy prior to surgical operation.
Follow-up was conducted every three to six months
for the first three years, and once a year thereafter. All patients
were followed up by out-patient review and telephone interviews.
The clinicopathological and follow-up findings were collected and
recorded in the database.
Surgery
All patients in the study underwent total or distal
gastrectomy, depending on the location and macroscopic appearance
of the tumor. Distal and total gastrectomies were performed
principally for tumors located in the lower third, middle, or upper
third of the stomach, and for tumors occupying the entire stomach.
The strategy for lymph node dissections was determined by using a
standardized technique according to the guidelines of the 2010
Japanese Classification of Gastric Cancer and Gastric Cancer
Treatment Guidelines, edited by the Japanese Gastric Cancer
Association (9), which consider No.
12a lymph node metastasis as regional progression.
In the present study, a clear and practical scope
definition of the No. 12a group lymph nodes of gastric cancer is
provided according to our clinical experiences and practices. Given
that the scope definition of No. 12a group lymph nodes is
undefinable according to the guidelines of AJCC/Union for
International Cancer Control and Japanese treatment guidelines,
none of the guidelines are able to describe the scope definition of
No. 12a group lymph nodes for gastric cancer (Table I and Fig.
1).
The surgical procedures of No. 12a node resection
were as follows. First, the ligamentum hepatoduodenale was exposed,
the gastroduodenum segment was stretched, and the ligamentum
hepatoduodenale was flattened. The anterior hepatoduodenal ligament
was opened to the confluence of the right and left hepatic
arteries. Secondly, the perivascular sheath along the hepatic
artery was opened to the upper border of the pancreas at the origin
of the proper hepatic artery, including the left side border
peritoneum fusion site of the ligamentum hepatoduodenale, also
including the lymphoid tissue. Finally, the perivascular sheath of
the portal vein was opened, and the lymphoid tissue at the front of
portal vein was cleared. An electrotome (Force FXTM-8C; Tyco
Healthcare Group LP, Boulder, CO, USA) was used in the whole
resection process for sharp dissection.
Statistical analysis
All statistical analyses were performed using IBM
SPSS software (v. 19.0; IBM, Armonk, NY, USA). The categorical
variables were compared by using the Chi-squared test or Fisher's
test. Survival curves were calculated using the Kaplan-Meier
method, and compared using the log-rank test. Logistic regression
analysis was used to assess the risk factors of No. 12a lymph node
metastasis. A Cox proportional hazard model was used to explore the
independent factors of survival status on the basis of the
variables selected in univariate analysis. P<0.05 was considered
to indicate a statistically significant value for each
analysis.
Results
Clinicopathological
characteristics
Among the 169 patients who underwent No. 12a lymph
node resection, 119 (70%) patients were men and 50 (40%) were
women. The mean age at diagnosis was 64.1±12 years, and follow-up
periods were between 0.9 and 77.5 months (median, 40 months). A
total number of 34 (20%) patients had an involvement of No. 12a
lymph nodes [No. 12a(+)]. The mean positive yield was
2.4 (range, 1–7) No. 12a lymph nodes. The data associated with the
clinical and pathological characteristics are shown in Table II. No differences were exhibited in
age, gender or tumor location between the No. 12a(+) patients and
the No. 12a(−) patients. No. 12a(+) patients did have significantly
higher clinicopathological parameters (P<0.05), including T
stage, and exhibited severe histological grade, a number of
metastatic lymph nodes, numerous, commonly intravascular, cancer
emboli, severe nerve invasion and large tumor size. All surgical
procedures were performed by the identical surgical team.
 | Table II.Comparison of clinical parameters in
patients with gastric cancer with or without No. 12a group lymph
node metastasis. |
Table II.
Comparison of clinical parameters in
patients with gastric cancer with or without No. 12a group lymph
node metastasis.
| Clinicopathological
parameters | No.
12a(−)a | No.
12a(+)b | N | P-value |
|---|
| Age (year) |
|
|
| 0.5422 |
|
<61 | 43 | 13 | 56 |
|
| ≥61 | 92 | 21 | 113 |
|
| Gender |
|
|
| 0.2927 |
| Male | 92 | 27 | 119 |
|
|
Female | 43 | 7 | 50 |
|
| Histological
grade |
|
|
| 0.0006e |
|
H+Mc | 58 | 4 | 62 |
|
|
L+Od | 77 | 30 | 107 |
|
| Tumor location |
|
|
| 0.5689 |
|
Upper | 53 | 12 | 65 |
|
|
Middle | 22 | 2 | 24 |
|
|
Lower | 60 | 20 | 80 |
|
| AJCC stage |
|
|
| 0.0063e |
| I+II | 30 | 1 | 31 |
|
|
III+IV | 105 | 33 | 138 |
|
| T stage |
|
|
| 0.0002e |
|
T1-T2 | 34 | 0 | 34 |
|
|
T3-T4 | 101 | 34 | 135 |
|
| Lymph node
metastasis |
|
|
| 0.0008e |
| No | 30 | 0 | 30 |
|
| Yes | 105 | 34 | 139 |
|
| Intravascular cancer
emboli |
|
|
| 0.0001e |
| No | 67 | 4 | 71 |
|
| Yes | 68 | 30 | 98 |
|
| Nerve invasion |
|
|
| 0.0001e |
| No | 84 | 6 | 90 |
|
| Yes | 51 | 28 | 79 |
|
| Tumor size |
|
|
| 0.0070e |
| <5
cm | 66 | 8 | 74 |
|
| ≥5
cm | 69 | 27 | 96 |
|
Significance of the survival outcome
of patients with No. 12a lymph nodes
A comparison between patients with or without No.
12a lymph node metastasis revealed that those with No. 12a lymph
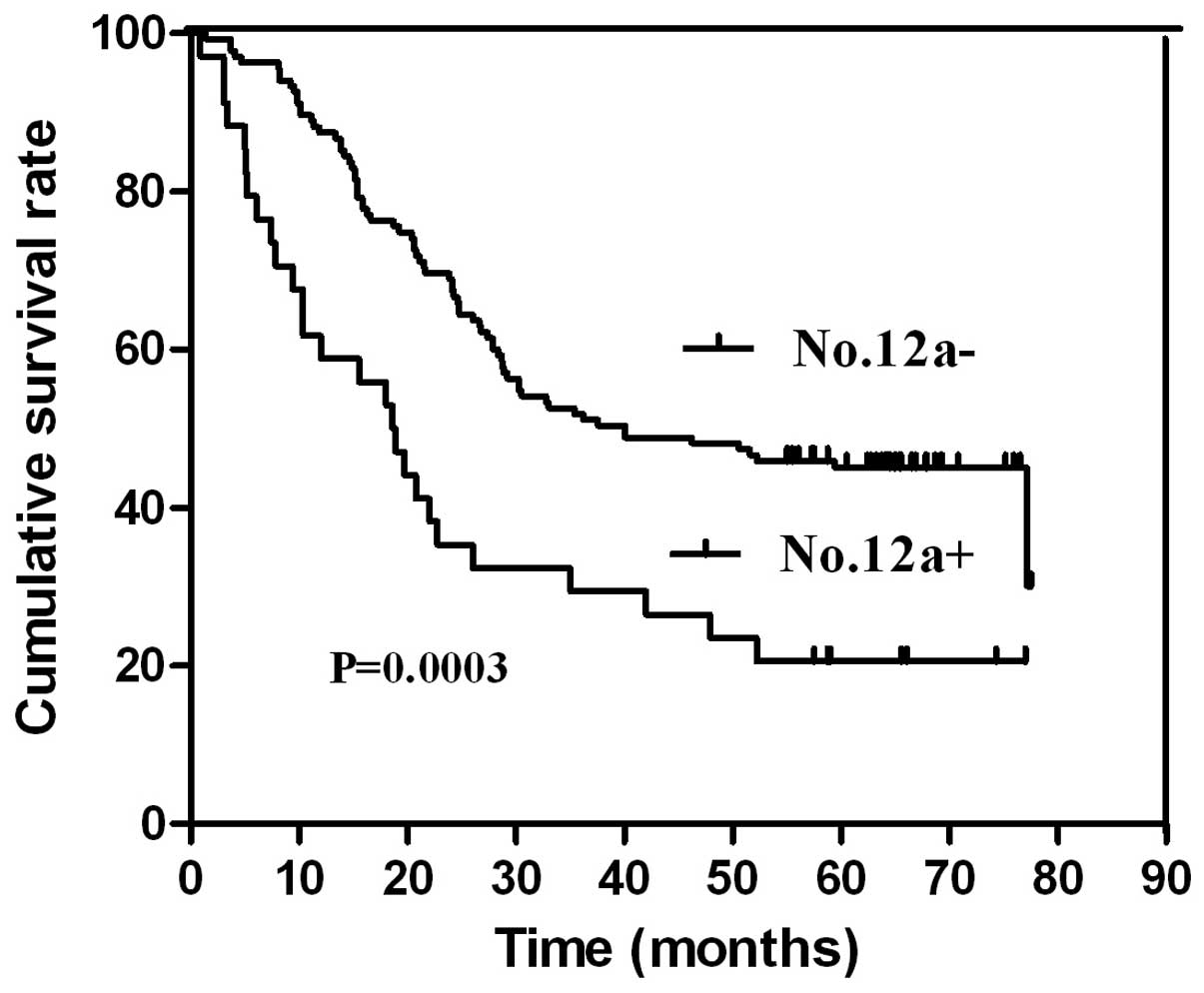
node metastasis had a markedly poorer survival outcome (Fig. 2). A similar result was found between
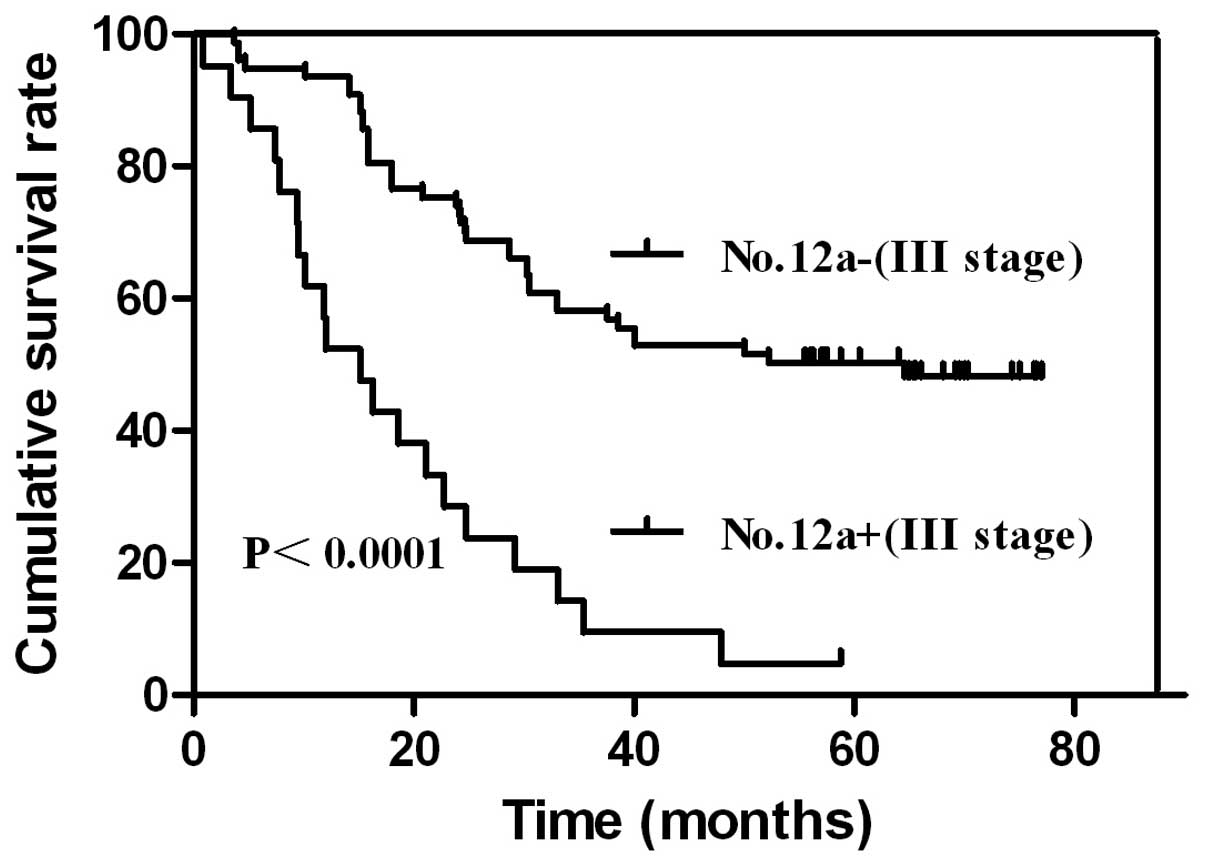
patients with or without No. 12a lymph node metastasis in stage III
patients (Fig. 3). However, in the
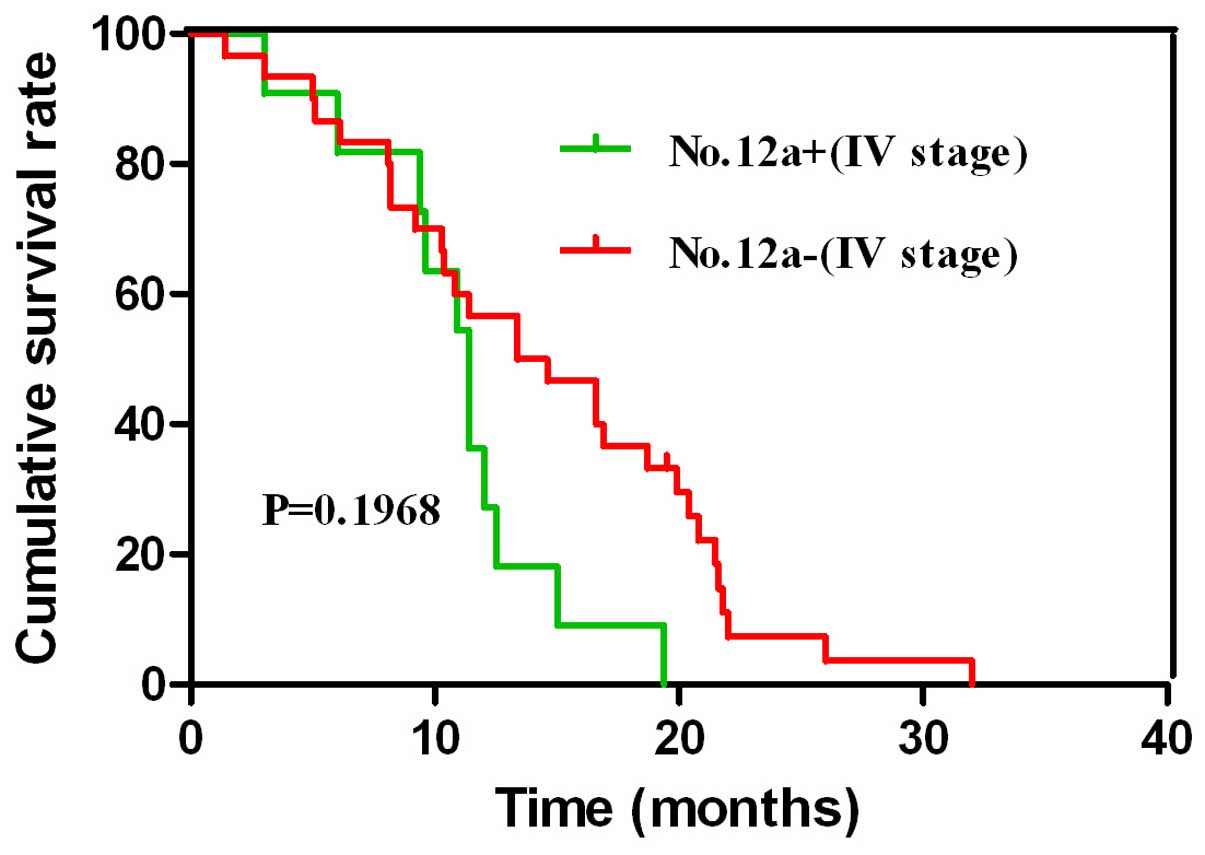
stage IV patients, no differences were observed in the survival
outcome between the No. 12a(+) group and the No. 12a(−) group
(Fig. 4). Univariate analysis was
performed for clinicopathological data that possibly affected
survival outcomes. Multivariate analysis was performed using the
variables that were significant in the univariate analysis. Cox
regression analysis revealed that the AJCC stage was the only
prognostic factor that was independently associated with an
unfavorable cumulative survival rate (Table III).
 | Table III.Univariate and multivariate analysis
of prognostic factors for the cumulative survival rate. |
Table III.
Univariate and multivariate analysis
of prognostic factors for the cumulative survival rate.
|
| Univariate
analysisa | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age | 1.01 | 0.994–1.027 | 0.219 |
|
|
|
| Gender,
male/female | 0.802 | 0.530–1.212 | 0.295 |
|
|
|
| Pathological
T-category (T1-T2/T3-T4) | 4.34 | 1.376–13.688 | 0.012b | 1.832 | 0.532–6.305 | 0.337 |
| AJCC stage
(I–II/III–IV) | 3.89 | 2.127–7.118 | 0.0b | 3.091 | 1.581–6.042 | 0.001b |
| Tumor
differentiation (moderately well differentiated/poorly
differentiated) | 0.721 | 0.458–1.136 | 0.158 |
|
|
|
| Tumor size
(<5/≥5 cm) | 1.152 | 0.790–1.680 | 0.462 |
|
|
|
| Tumor location
(upper/middle/low) | 0.965 | 0.769–1.212 | 0.761 |
|
|
|
| No. 12a metastasis
status | 1.90 | 1.221–2.956 | 0.004b | 1.458 | 0.929–2.288 | 0.101 |
| Intravascular
cancer emboli | 1.497 | 1.021–2.195 | 0.039b | 1.137 | 0.766–1.690 | 0.524 |
| Nerve invasion | 1.417 | 0.961–2.089 | 0.079 | 0.933 | 0.620–1.402 | 0.738 |
Influence factors of No. 12a lymph
node metastasis
Logistic regression analysis revealed that tumor
location, the AJCC stage, intravascular cancer emboli and nerve
invasion were associated with No. 12a lymph node metastasis
(Table IV).
 | Table IV.Univariate logistic regression
analysis of No. 12a lymph node metastasis. |
Table IV.
Univariate logistic regression
analysis of No. 12a lymph node metastasis.
| Factor | OR | 95% CI | P-value |
|---|
| Age | 1.034 | 0.998–1.070 | 0.064 |
| Gender | 0.797 | 0.345–1.839 | 0.595 |
| Histological grade
(H+Ma/P+Ob) | 0.694 | 0.278–1.733 | 0.434 |
| Tumor location
(upper/middle/low) | 0.161 | 4.173–5.535 |
<0.001c |
| AJCC stage
(I+II/III+IV) | 11.96 | 1.577–90.686 | 0.016c |
| T stage
(T1+T2/T3+T4) | – | – | 0.999 |
| Intravascular
cancer emboli | 2.512 | 1.112–5.676 | 0.027c |
| Nerve invasion | 2.462 | 1.135–5.342 | 0.023c |
| Tumor size | 0.969 | 0.453–2.075 | 0.936 |
Discussion
Gastric cancer remains one of the most common causes
of cancer-associated mortality in the world. Surgical resection is
a curative treatment that is available for advanced cases, and
lymphadenectomy is an important part of curative resection
(12). Lymph node radical dissections
for advanced gastric cancer are theoretically able to increase the
patient survival rate. Thus, D2 dissection is a standard procedure
for patients with gastric cancer. Since the 1990s, this procedure
has been increasingly employed by surgeons to treat gastric cancer
(7,8,13).
Two major staging systems are used for gastric
cancer: The Japanese treatment guidelines, and the AJCC guidelines.
The majority of East Asian countries use both guidelines, although
the AJCC guidelines are used worldwide. Both the 6th and 7th
editions of the AJCC guidelines for gastric cancer base D2
lymphadenectomy on the tumor position.
However, a number of novel and carefully considered
changes are present in the 7th edition of the AJCC guidelines for
gastric cancer, for example, the exclusion of No. 12a lymph node
dissection from D2 lymphadenectomy. However, these changes were not
explained properly. This issue provided the impetus to conduct the
present study.
On critically assessing the available information,
no clear definition was identified to exist for No. 12a lymph
nodes. Therefore, in the present study, a clear and practical
definition of No. 12a group lymph nodes has been provided according
to our clinical experiences and practices. The clinical importance
and survival outcomes of patients with gastric cancer with No. 12a
lymph node metastasis following D2 lymphadenectomy were also
evaluated.
The data in the present study revealed that No. 12a
lymph node metastasis occurred in 20% (34/169) of the patients.
This study also revealed that No. 12a lymph node metastasis was
correlated with the histological grade, AJCC stage, T stage, lymph
node metastasis, intravascular cancer emboli, nerve invasion and
tumor size of the patients with gastric cancer. These results were
in agreement with those of Shirong et al (14). In the present study, the rate of
metastasis for the No. 12a lymph nodes was higher, predominantly
since the patients in our study were diagnosed with advanced-stage
disease. The percentage of stage III and IV patients with gastric
cancer was 81.67% (138/169).
The present study demonstrated that survival
outcomes were different between cases of No. 12a lymph node
metastasis and those of lymph node involvement in the 7th edition
AJCC-defined D2 lymphadenectomy region. Furthermore, the survival
outcome was poorer in patients with No. 12a lymph node metastasis
compared with those of No. 12a lymph node metastasis in stage III.
However, in stage IV patients with gastric cancer, survival
outcomes were similar between cases of No. 12a lymph node
metastasis and those of distant metastasis. In the current study,
No. 12a lymph node metastasis was linked with poor malignant tumor
behavior and an advanced tumor stage. Therefore, the present
results support the hypothesis that No. 12a group lymph node
metastasis should be considered as distant lymph node metastasis,
and this concurs with the perspective of the 7th edition AJCC
regarding No. 12a lymph node metastasis. The results of the present
study on No. 12a lymph node metastasis contradict those of Shirong
et al (14), who proposed that
No. 12a lymph node metastasis should be considered as regional
lymph node metastasis.
In the present study, Cox regression analysis
demonstrated showed that the AJCC stage was independently
associated with an unfavorable cumulative survival rate. Logistic
regression analysis revealed that tumor location, the AJCC stage,
intravascular cancer emboli and nerve invasion were associated with
No. 12a lymph node metastasis.
However, the limitations of the present study
include its retrospective design. The number of patients in this
study was lower in comparison with those in other studies, since
the scope of lymphadenectomy was strict and normative according to
the scope definition of No. 12a lymph nodes. The scientificity and
rationality of our hypothesis regarding No. 12a lymph nodes require
further supporting evidence to substantiate them, and more
randomized controlled trial studies will be required in the
future.
In conclusion, the present study has demonstrated,
to the best of our knowledge for the first time, a clear and
practical scope definition of No. 12a group lymph nodes of gastric
cancer, according to our clinical experiences and practices
(Table I and Fig. 1). The survival outcome of patients
with gastric cancer and No. 12a lymph node metastasis was poorer
compared with that of patients with No. 12a lymph node metastasis.
The results were similar in stage III patients with gastric cancer.
However, the survival outcome of patients was similar with or
without No. 12a lymph node metastasis in stage IV gastric cancer.
Therefore, the present data suggest that No. 12a lymph node
metastasis is associated with distant metastasis, and they are
supportive of the 7th edition AJCC gastric cancer guidelines, which
have correctly classified No. 12a lymph node metastasis as distant
metastasis.
Acknowledgements
The present study was supported by the Key Project
of Science and Technology Research Program in Fujian Province (no.
2012B002), the Fujian Provincial Natural Science Foundation (no.
2014J01309), the Backbone Teacher Project of Fujian Medical
University (no. JGG200716), a China Non Intervention Gastric Cancer
Registration Survey Clinical Research Project (no. QT-201403), a
Ministry of Health Medicine Science and Technology Development and
Research grant (no. W2013FZ08) and the National Clinical Key
Specialty Construction Project (General Surgery) of China.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012v1.0. Cancer incidence and mortality
worldwide: IARC CancerBase (no. 11. Lyon). 2013.http://globocan.iarc.frAccessed. April
25–2014
|
|
2
|
Carneiro F: Stomach Cancer. Steward B and
Wild CP: WorldCancer Report, IARC. Lyon: 383–391. 2014.
|
|
3
|
Aurello P, D'Angelo F, Nigri G, Bellagamba
R, Cicchini C, Ruzzetti R and Ramacciato G: Comparison between site
N-category and number N-category for nodal staging in carcinoma of
the gastroesophageal junction: Our experience and literature
review. Am Surg. 72:118–123. 2006.PubMed/NCBI
|
|
4
|
Sayegh ME, Sano T, Dexter S, Katai H,
Fukagawa T and Sasako M: TNM and Japanese staging systems for
gastric cancer: How do they coexist? Gastric Cancer. 7:140–148.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sobin LH and Wittekind C: UICC: TNM
classification of malignant tumours (7th). Wiley-Liss. New York,
NY: 2010.
|
|
6
|
Edge SBBD, Compton CC, Fritz AG, Greene FL
and Trotti A: AJCC Cancer Staging Manual (7th). Springer. New York,
NY: 2010.
|
|
7
|
Degiuli M, Sasako M, Ponti A, Vendrame A,
Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G and Morino M:
Italian Gastric Cancer Study Group: Randomized clinical trial
comparing survival after D1 or D2 gastrectomy for gastric cancer.
Br J Surg. 101:23–31. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang L, Yang KH, Guan QL, Zhao P, Chen Y
and Tian JH: Survival and recurrence free benefits with different
lymphadenectomy for resectable gastric cancer: A meta-analysis. J
Surg Oncol. 107:807–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bilimoria KY, Bentrem DJ, Ko CY, Ritchey
J, Stewart AK, Winchester DP and Talamonti MS: Validation of the
6th edition AJCC pancreatic cancer staging system: Report from the
national cancer database. Cancer. 110:738–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Japanese, Biliary, Tract, Cancers and
Classification: Japanese classification of Biliary tract carcinoma.
English edition Biliary Tract Cancer. 2015.
|
|
12
|
Furukawa H, Imamura H and Kodera Y: The
role of surgery in the current treatment of gastric carcinoma.
Gastric Cancer. 5(Suppl 1): S13–S16. 2002. View Article : Google Scholar
|
|
13
|
Tsubono Y and Hisamichi S: Screening for
gastric cancer in Japan. Gastric Cancer. 3:9–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirong C, Jianhui C, Chuangqi C, Kaiming
W, Xinhua Z, Wu S and Yulong H: Survival of proper hepatic artery
lymph node metastasis in patients with gastric cancer: Implications
for D2 lymphadenectomy. PloS One. 10:e01189532015. View Article : Google Scholar : PubMed/NCBI
|