Introduction
Since anaplastic pancreatic carcinoma (ANPC) is rare
and accounts for only 2–7% of all pancreatic carcinoma cases, its
clinical features and surgical outcomes remain to be elucidated
(1,2).
A total of three pathological subtypes of ANPC exist: Spindle cell
carcinoma, giant cell carcinoma, and pleomorphic carcinoma.
Surgical resection is the only curative therapy for patients with
ANPC since no effective systemic chemotherapy or other
interventions are available; however, long-term survival remains to
be achieved in patients with ANPC even following curative surgery
(1,3).
ANPC is often associated with a delay in clinical presentation as a
result of its asymptomatic nature until the tumor has progressed to
an advanced stage. In several reports that have been published
since the early 1900s, ANPC was referred to as ‘giant-cell tumor’,
‘undifferentiated carcinoma’ with or without osteoclast-like giant
cells and ‘pleomorphic carcinoma’ of the pancreas (3–6).
Establishing the precise pre-operative diagnosis is
difficult due to the radiological findings being atypical and
similar to those of gastrointestinal stromal tumors, mucinous cyst
adenocarcinomas and pancreatic carcinomas. Upon admission, the
present patient's tumor was already huge and revealed enhanced rims
with hypodense lesions on computed tomography (CT) scans, which
appears to be a common radiological feature of ANPCs (1).
Of the three subtypes, spindle cell carcinoma is the
most aggressive subtype of sarcomatoid carcinoma and currently no
effective systemic chemotherapies or other interventions are
available. According to previous reports, two patients with ANPC
with rhabdoid features had dismal prognoses following surgical
intervention (7,8). The present study described a case of
spindle cell ANPC that exhibited aggressive growth following
emergency curative surgery.
Case report
A 74-year-old woman without any previous medical
history was admitted to our hospital in April 2015. Physical
examination revealed a firm tumor located in her left upper
quadrant. Abdominal ultrasonography detected a giant cystic tumor
containing high and low echoic lesions in the body and tail of the
pancreas. Admission laboratory tests revealed that the serum levels
of carbohydrate antigen 19-9, carcinoembryonic antigen and DUPAN-2
were elevated (78.0, 36.6 and 1,200 U/ml, respectively). On
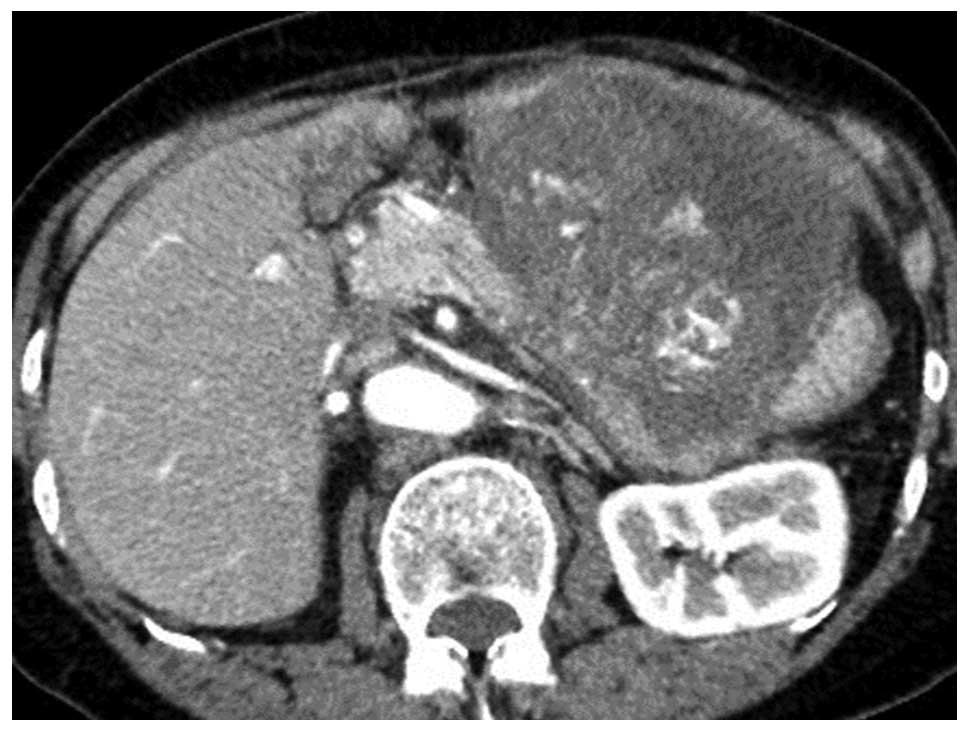
contrast-enhanced CT scanning, the tumor occupying the left upper
and lower abdomen was measured to be 9.5×8.0 cm. This tumor
consisted of multilocular cystic components, both the rim and core
of the tumor were strongly enhanced on the arterial phase CT images
(Fig. 1). A dilatation of the distal
main pancreatic duct was evident, however, no distant metastases,
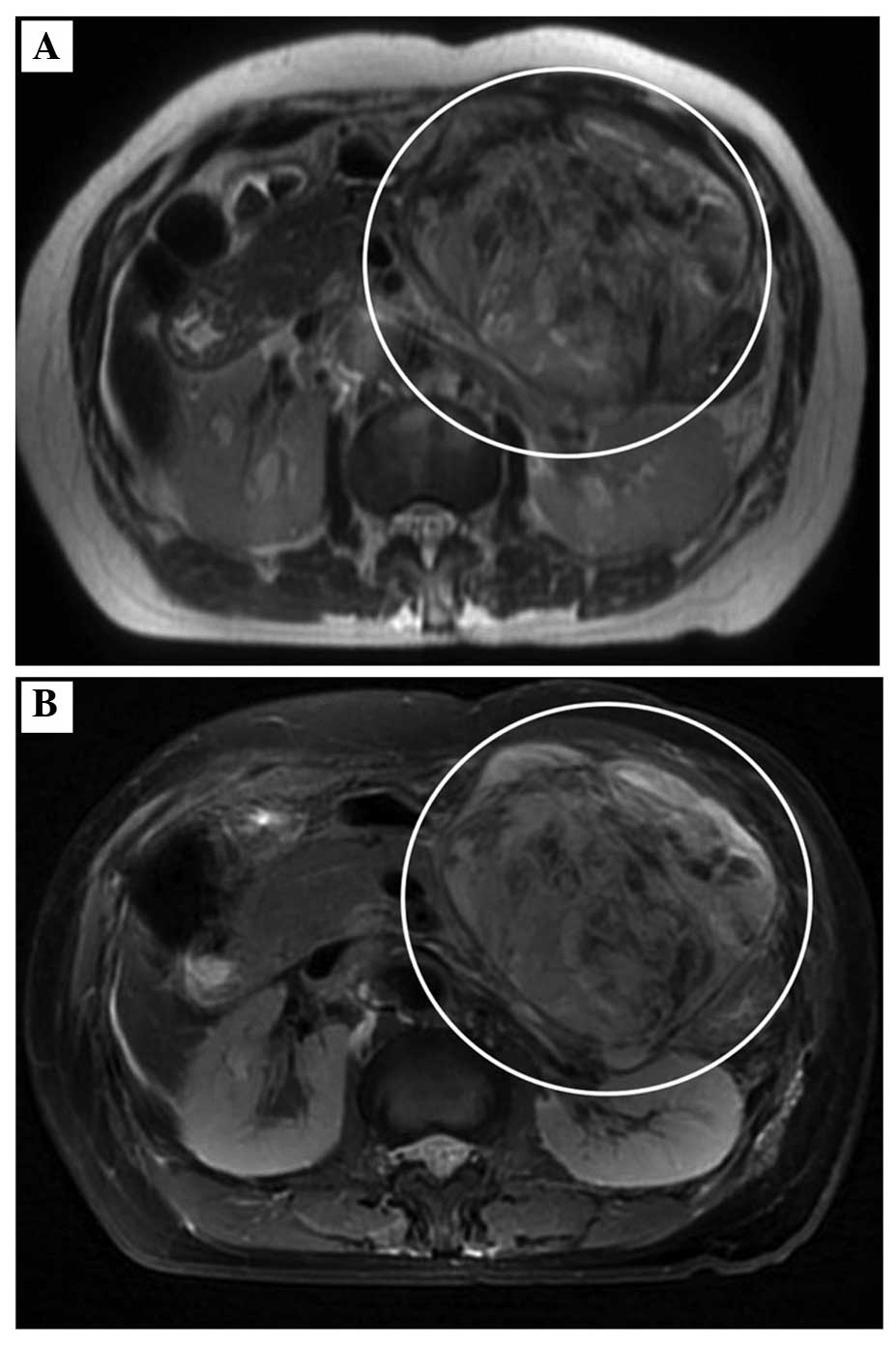
lymph node swelling or ascites were detected. The solid tumor
exhibited low signal intensity on T1-weighted magnetic resonance
imaging (MRI) and relatively high signal intensity on T2-weighted
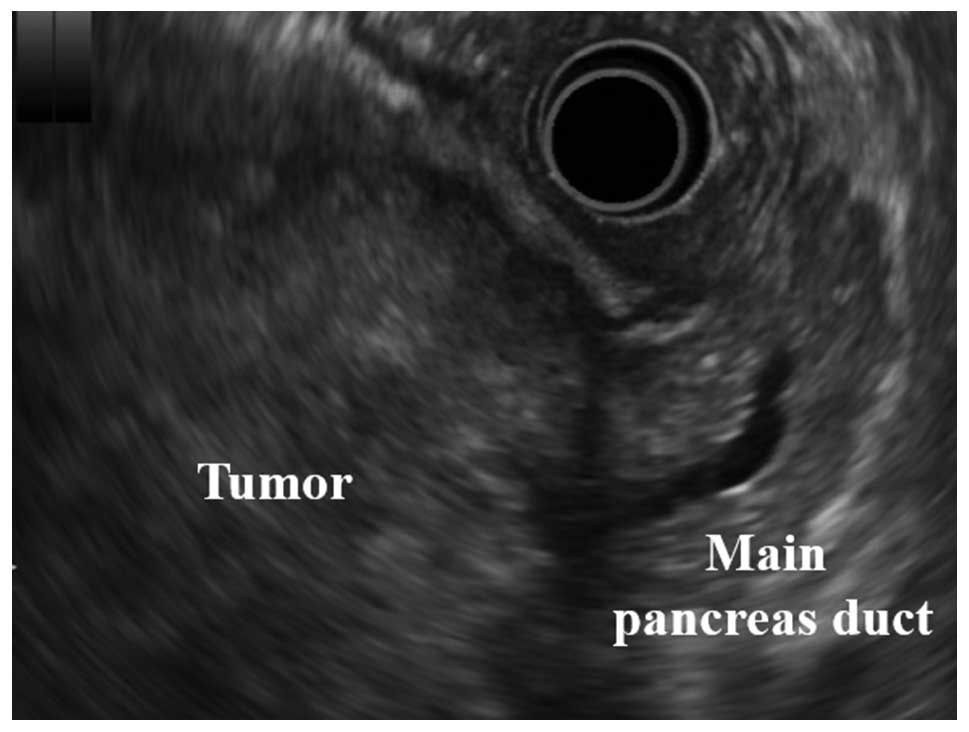
MRI (Fig. 2). Intraductal
ultrasonography demonstrated a huge solid tumor derived from the
body of the pancreas and located within a clear margin from the
stomach (Fig. 3).
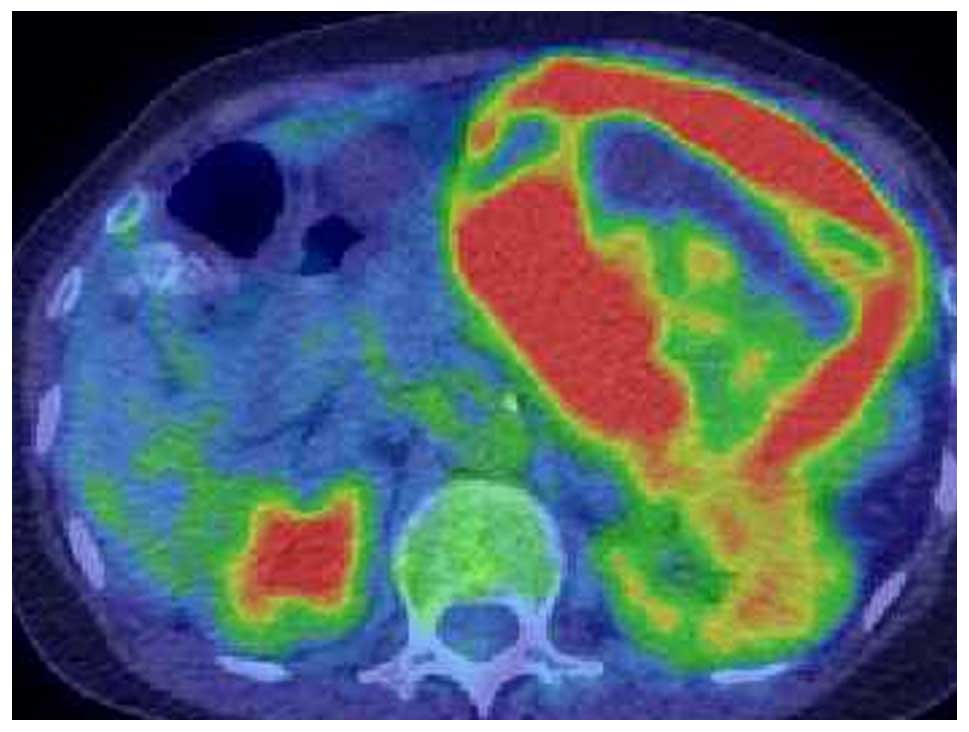
18F-fluorodeoxyglucose (FDG)-positron emission
tomography/CT identified no distant metastasis, and FDG
accumulation was detected only in the tumor lesion (Fig. 4). The differential diagnoses were
pancreatic adenocarcinoma, gastrointestinal stromal tumor,
endocrine cell carcinoma or solid pseudopapillary neoplasm of the
pancreas. At 3 weeks after the initial admission of the patient,
massive ascites suddenly emerged and the tumor increased in from a
size of 9.5 cm to 11 cm in the maximal diameter. The patient
experienced an increase in acute abdominal pain, which was
uncontrollable by analgesic medications. During an emergency
laparotomy, bloody ascites of 1,700 ml was observed; however,
peritoneal dissemination or liver metastasis was not detected. A
distal pancreatectomy and a splenectomy with regional lymph node
dissection were performed. The operation lasted 4 h 13 min. Blood
loss during the operation was 1,300 ml and the bloody ascites
volume was 1,700 ml.
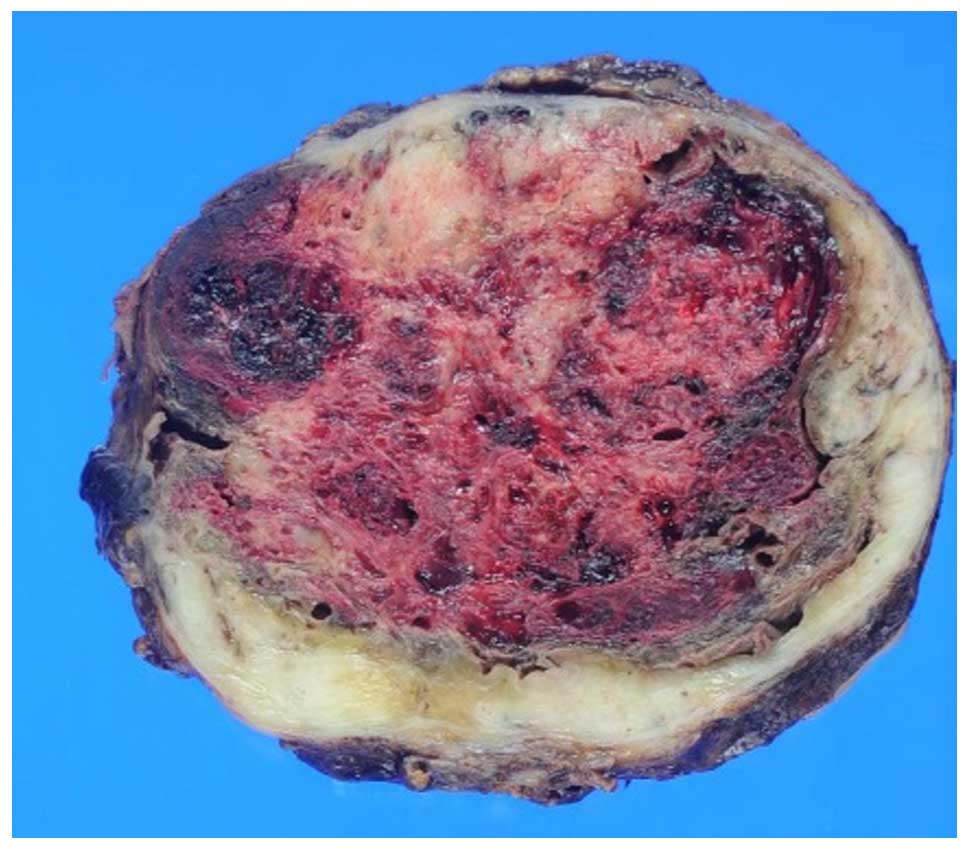
On macroscopic observation, the tumor was 11×12 cm,
and its appearance was elastic, hard and a white mass (Fig. 5). All specimens were processed in a
routine manner for paraffin embedding and 5 µm-thick sections were
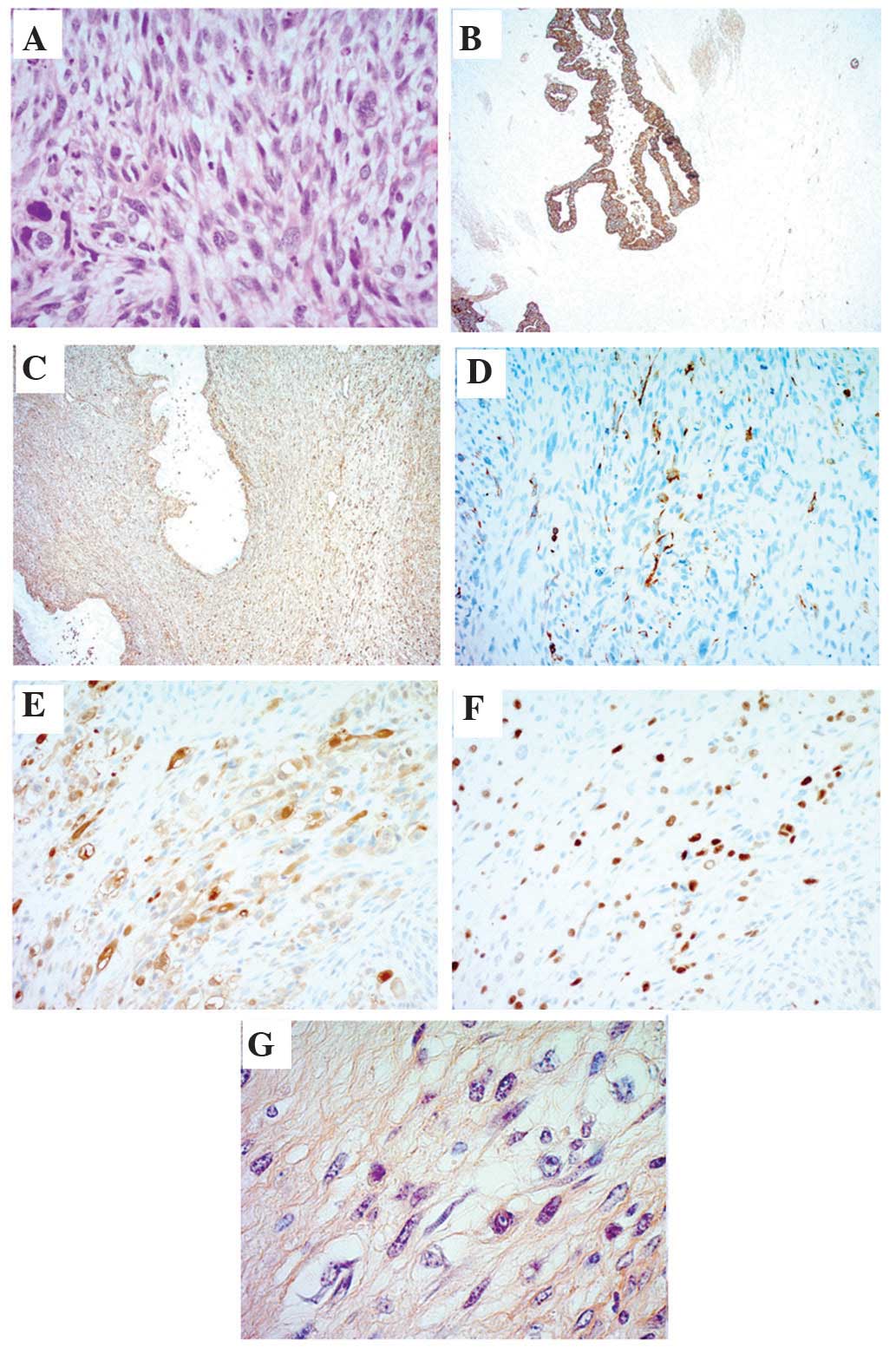
cut and stained with hematoxylin and eosin. Histopathological
analysis of the sections revealed that the tumor cells had acquired
dedifferentiated rhabdoid features and were positive for
phosphotungstic acid-hematoxylin. Additionally, the tumor cells
were positive for phosphotungstic acid-hematoxylin (PTAH) stain,
myoglobin (cat. no. H0309; Nichilei Biosciences, Inc., Tokyo,
Japan), myogenin (F5D; cat. no. 1328706B; Dako, Carpinteria, CA,
USA), vimentin (V9; cat. no. H1402; Dako) and cytokeratin 5.2
(CAM52; cat. no. D04930; Roche Diagnostics, Indianapolis, IN, USA;
Fig. 6). Therefore, a diagnosis of
spindle cell ANPC with rhabdoid features was made based on the
pathological findings. The patient was discharged 14 days
post-operation without any complications. The patient succumbed to
mortality 8 months following the surgery while undergoing systemic
adjuvant chemotherapy.
Discussion
Spindle cell ANPC has been reported to have the
worst prognosis of the three ANPC subtypes (3). The presence of rhabdoid features in ANPC
is extremely rare; only two such cases have been reported to date
(7,8).
ANPC accounts for 2–7% of all pancreatic carcinoma cases. The
present case exhibited an atypical clinical presentation and
radiological findings, which allowed the present study to
distinguish ANPC from gastrointestinal stromal tumor, solitary
pseudopapillary neoplasms and pancreatic adenocarcinoma (1). Rapid tumor progression associated with
massive ascites and uncontrollable abdominal pain occurred in the
present case. Distal pancreatectomy and splenectomy were
successfully performed to excise the giant tumor. Establishing the
precise diagnosis was difficult due to the atypical radiological
findings and tumor rarity. Although a huge tumor of the pancreas
can be treated via surgical resection, the prognosis is poor even
following surgery. Whenever possible, radical resection is
recommended.
Although multiple case reports on ANPC have been
published, the clinical presentation and surgical outcomes remain
to be well investigated owing to the rarity of this disease
(3,9–12). Upon
admission of the patient, the radiological findings on
contrast-enhanced CT was a huge tumor with a well-enhanced rim and
hypodense areas in the center. On T1-weighted MRI, the tumor
exhibited a low signal intensity, however, on T2-weighted MRI, the
intensity of the signal was high.
Strobel et al (1) reported that ANPC is an aggressive type
of pancreatic cancer with a dismal prognosis (1). Whenever possible, resection must be
attempted, as it is the only treatment associated with a favorable
prognosis. Although the clinical presentation and radiological
findings are similar to those of pancreatic carcinoma, despite the
lack of a precise diagnosis, curative surgery, whenever possible,
must not be postponed. According to previous reports, obtaining
pre-operative tissue samples does not change the therapeutic
approach and is not advisable if the neoplasm appears
resectable.
Neither an effective chemotherapy nor a standard
regimen has been established for ANPC. Neoadjuvant chemotherapy was
reported to be effective in one case, however, a standard regimen
was not established (13). Wakatsuki
et al (12) reported that a
complete response was achieved following treatment with paclitaxel
(PTX) (12). The present patient was
treated with a nab-PTX and gemcitabine regimen, which failed to
prevent multiple live metastases and peritoneal disseminations.
PTX, a microtubule-stabilizing agent, has proven effective in
treating several types of cancer, including breast, lung and
ovarian. PTX exerts a particularly strong antitumor activity
against certain types of sarcomas including angiosarcoma, Kaposi
sarcoma and carcinosarcoma of the uterus and heart (14–16).
Strobel et al (1) compared clinical features and surgical
outcomes of ANPC and pancreatic ductal adenocarcinoma. In the ANPC
group, the duration of survival was significantly greater following
R0/R1 resection compared with after palliative treatment. On the
basis of the results of the previous study, the authors recommend
that patients with ANPC be operated on whenever a potentially
curative resection is possible since established systemic
chemotherapy or alternative therapy is available. Among the several
subtypes of ANPC, the spindle cell type exhibited the worst
prognosis even following curative surgery; the prognosis was
particularly poor in the present case of ANPC associated with
rhabdoid features.
Immunohistochemistry revealed that the present
patient's tumor was positive for cytokeratin 5.2, vimentin, desmin,
myoglobin, myogenin and PTAH. Kane et al (3) reviewed the immunohistochemistry results
of sarcomatoid components from previous reports and their results
are consistent with the present positive findings for desmin,
vimentin and myogenin (3). Notably,
PTAH positivity, which represents cell dedifferentiation into
striated muscle, was detected only in the present case. These
phenotypic changes suggested that the epithelial cancer cells had
transformed into mesenchymal cancer cells. The mechanism of such a
transformation may be owing to the presence of cancer stem cells or
the dedifferentiation of tumor cells into sarcoma cells.
In conclusion, ANPC is a rare and aggressive variant
of pancreatic cancer. Spindle cell ANPC is associated with a
particularly dismal prognosis. The only typical radiological
feature of ANPC is a large tumor with an enhanced rim and a
hypodense area in the center. As surgical resection is the only
intervention that results in long-term survival in patients with
ANPC, it must be attempted whenever possible. Further studies are
required to clarify the mechanism of the aggressiveness of this
type of cancer.
References
|
1
|
Strobel O, Hartwig W, Bergmann F, Hinz U,
Hackert T, Grenacher L, Schneider L, Fritz S, Gaida MM, Büchler MW
and Werner J: Anaplastic pancreatic cancer: Presentation, surgical
management, and outcome. Surgery. 149:200–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoorens A, Prenzel K, Lemoine NR and
Klöppel G: Undifferentiated carcinoma of the pancreas: Analysis of
intermediate filament profile and Ki-ras mutations provides
evidence of a ductal origin. J Pathol. 185:53–60. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kane JR, Laskin WB, Matkowskyj KA, Villa C
and Yeldandi AV: Sarcomatoid (spindle cell) carcinoma of the
pancreas: A case report and review of the literature. Oncol Lett.
7:245–249. 2014.PubMed/NCBI
|
|
4
|
Fabre J, Planques J, Bouissou H and
Sendrail-Pesque M: Sarcomatoid carcinoma of the pancreas. Toulouse
Med. 62:85–98. 1961.(In French). PubMed/NCBI
|
|
5
|
Alguacil-Garcia A and Weiland LH: The
histologic spectrum, prognosis, and histogenesis of the sarcomatoid
carcinoma of the pancreas. Cancer. 39:1181–1189. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ackerman NB, Aust JC, Bredenberg CE,
Hanson VA Jr and Rogers LS: Problems in differentiating between
pancreatic lymphoma and anaplastic carcinoma and their management.
Ann Surg. 184:705–708. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda N, Iwamura S, Fujishima N, Ohara M,
Hirouchi T, Mizuno K, Hayashi Y and Lee GH: Anaplastic carcinoma of
the pancreas with rhabdoid features and hyaline globule-like
structures. Med Mol Morphol. 40:168–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuroda N, Sawada T, Miyazaki E, Hayashi Y,
Toi M, Naruse K, Fukui T, Nakayama H, Hiroi M, Taguchi H and Enzan
H: Anaplastic carcinoma of the pancreas with rhabdoid features.
Pathol Int. 50:57–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sano M, Homma T, Hayashi E, Noda H, Amano
Y, Tsujimura R, Yamada T, Quattrochi B and Nemoto N:
Clinicopathological characteristics of anaplastic carcinoma of the
pancreas with rhabdoid features. Virchows Arch. 465:531–538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okazaki M, Makino I, Kitagawa H, Nakanuma
S, Hayashi H, Nakagawara H, Miyashita T, Tajima H, Takamura H and
Ohta T: A case report of anaplastic carcinoma of the pancreas with
remarkable intraductal tumor growth into the main pancreatic duct.
World J Gastroenterol. 20:852–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiogi M, Kobayashi T, Yasuno M and
Tanaka M: Anaplastic carcinoma of the pancreas mimicking submucosal
gastric tumor: A case report of a rare tumor. Case Rep Med.
2013:5232372013.PubMed/NCBI
|
|
12
|
Wakatsuki T, Irisawa A, Imamura H,
Terashima M, Shibukawa G, Takagi T, Takahashi Y, Sato A, Sato M,
Ikeda T, et al: Complete response of anaplastic pancreatic
carcinoma to paclitaxel treatment selected by chemosensitivity
testing. Int J Clin Oncol. 15:310–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones TS, Jones EL, McManus M, Shah R and
Gajdos C: Multifocal anaplastic pancreatic carcinoma requiring
neoadjuvant chemotherapy and total pancreatectomy: Report of a
case. JOP. 14:289–291. 2013.PubMed/NCBI
|
|
14
|
Otsuki A, Watanabe Y, Nomura H, Futagami
M, Yokoyama Y, Shibata K, Kamoi S, Arakawa A, Nishiyama H, Katsuta
T, et al: Paclitaxel and carboplatin in patients with completely or
optimally resected carcinosarcoma of the uterus: A phase II trial
by the Japanese uterine sarcoma group and the tohoku gynecologic
cancer unit. Int J Gynecol Cancer. 25:92–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brambilla L, Romanelli A, Bellinvia M,
Ferrucci S, Vinci M, Boneschi V, Miedico A and Tedeschi L: Weekly
paclitaxel for advanced aggressive classic Kaposi sarcoma:
Experience in 17 cases. Br J Dermatol. 158:1339–1344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ram Prabu MP, Thulkar S, Ray R and Bakhshi
S: Primary cardiac angiosarcoma with good response to Paclitaxel. J
Thorac Oncol. 6:1778–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|