Introduction
Malignant ovarian germ cell tumors (MOGCTs) are
rare, accounting for ~5% of all ovarian malignancies (1). These tumors mainly affect girls and
young women and are usually diagnosed at early stages. Bilateral
involvement in MOGCT patients is very rare, with a reported
prevalence of 4.3–6.9% (2,3). It is crucial to carefully consider the
treatment modalities, as the affected patients are usually young
and strongly wish to preserve their fertility. Due to the high
sensitivity of this type of tumor to chemotherapy, the National
Comprehensive Cancer Network of the United States has unequivocally
suggested in their guidelines (version 2.2015) that
fertility-sparing surgery should be considered, even for patients
with advanced-stage disease (4).
However, for cases with bilateral involvement, preserving fertility
may be associated with increased risk, as cystectomy increases the
possibility of residual lesions. Due to the rarity of these tumors,
published related articles in the English literature are sparse. An
Italian study assessing multicenter data over 20 years aimed to
investigate this subject, but only found 8 patients with bilateral
involvement, in 4 of whom fertility was preserved (5).
The histological heterogeneity of MOGCTs adds to the
complexity of their management. MOGCTs may be divided into
dysgerminomas and non-dysgerminomas, of which the most common types
are yolk sac tumor, immature teratoma and mixed germ cell tumor.
Embryonal carcinoma, non-gestational choriocarcinoma and
polyembryoma are rare (4). The grade
of malignancy differs between various histological types. The
reported 5-year survival rates are also different: For bilateral
dysgerminoma, immature terotoma and mixed germ cell tumor, the
reported survival rates were 96, 94 and 87%, respectively (2).
Due to the scarcity of bilateral MOGCTs and the
varied histological types, it may be difficult to reach a
conclusion on whether conservative surgery (cystectomy) increases
the risks in these patients. The Obstetrics and Gynecology Hospital
of Fudan University (Shanghai, China) is one of China's biggest
tertiary referral centers. The aim of this study was to
retrospectively analyze the prevalence, clinical characteristics,
management and outcome of patients affected by bilateral MOGCTs in
this hospital, in order to accumulate more information on this
subject. We also conducted a review of the available literature to
help elucidate this issue.
Patients and methods
Patient selection criteria
Patients meeting the following criteria were
included in the study: i) Histologically proven MOGCT; ii) primary
surgery performed at the Obstetrics and Gynecology Hospital of
Fudan University between January, 2001 and December, 2014. A total
of 130 patients fitting the requirements were identified, of whom 8
exhibited bilateral involvement. Patient data, including age at
diagnosis, presenting complaint, ultrasonography (USG) findings,
serum tumor markers, surgical details, stage and histological type
of tumor, adjuvant chemotherapy and follow-up data, including
recurrence or death, were retrospectively collected from the
hospital medical records. The study protocol was approved by the
Institutional Review Board of the Obstetrics and Gynecology
Hospital of Fudan University. Written informed consent was acquired
from the patients.
Histological classification and
staging
The histological diagnosis was based on the
International Classification of Diseases for Oncology, 3rd edition,
of the World Health Organization (6).
Staging was performed according to the International Federation of
Gynecology and Obstetrics (FIGO) classification of ovarian tumors
(7). The complete staging procedure
included the following steps: i) Collection of ascitic fluid or
washings of the peritoneal cavity; ii) careful examination of the
peritoneum, biopsy and excision of any nodules; iii) omentectomy;
and iv) sampling or excision of the pelvic lymph nodes.
Fertility-sparing surgery is defined as the preservation of the
uterus and at least part of a unilateral ovary, whereas radical
surgery is defined as bilateral salpingo-oophorectomy (BSO), with
or without total hysterectomy.
Statistical analysis
Statistical analysis was performed with SPSS
software, version 16.0 (SPSS Inc., Chicago, IL, USA). Overall
survival (OS) was defined as the time interval from the date of
primary surgery to death or last visit (months). Disease-free
survival (DFS) was defined as the time interval from the date of
primary surgery to recurrence or last disease-free visit (months).
The Kaplan-Meier method was used to calculate survival and
comparisons between survival rates were performed with the
log-rank test. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Patient characteristics
Between January, 2001 and December, 2014, a total of
130 patients were pathogically diagnosed with MOGCTs and underwent
primary surgery at our hospital. The 5-year OS and DFS of all 130
patients were 96.3 and 90.5%, respectively. Eight (6.2%) of the
patients exhibited bilateral involvement. The demographic and
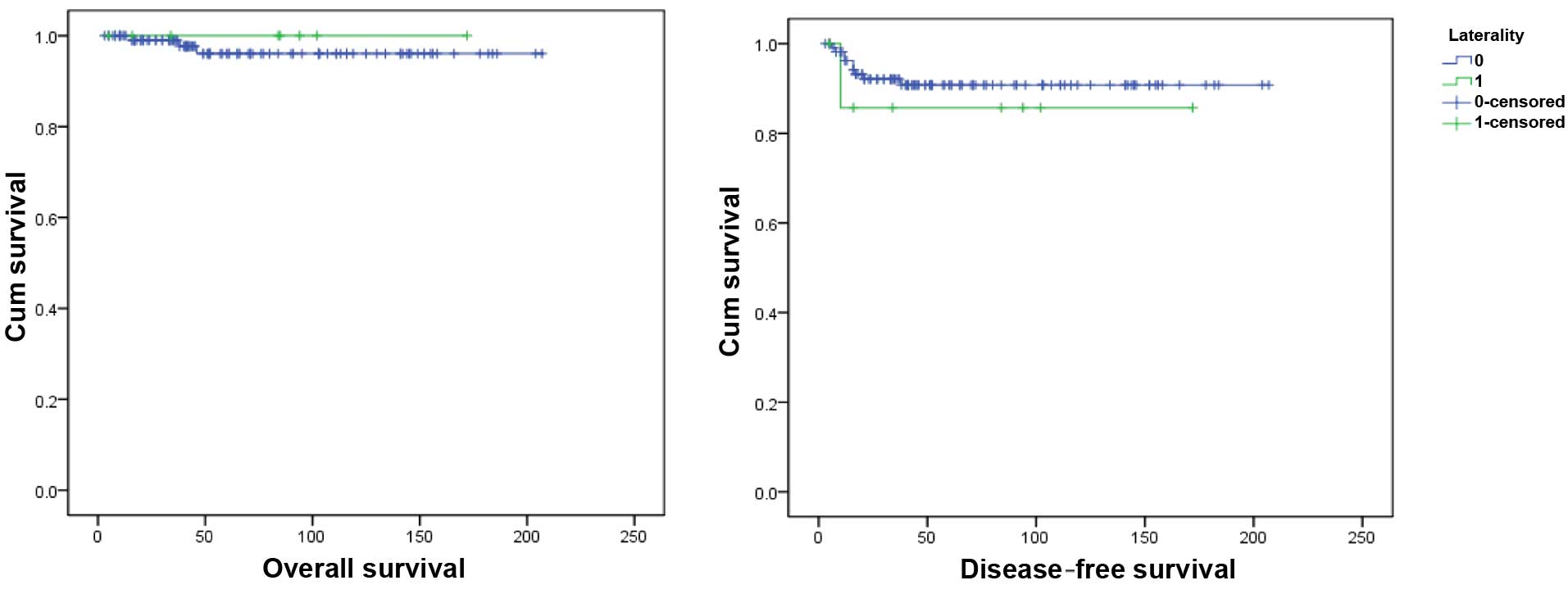
clinicopathological characteristics are listed in Table I. The univariate analysis demonstrated
that there was no significant difference in OS and DFS between the
unilateral and bilateral groups (P=0.639 and 0.563, respectively).
The survival curves are presented in Fig.
1.
 | Table I.Patient demographic and clinical
information. |
Table I.
Patient demographic and clinical
information.
| Case | Age (years) | Type | FIGO stage | Surgical method | Operative range | Fertility
sparing | Complete staging | Chemotherapy
(cycles) | Recurrence |
|---|
| 1 | 18 | Dysgerminoma | IC | Laparotomy | BSO + TAH + OT + LND
+ AT | No | Yes | PVB (8) | No |
| 2 | 18 | Dysgerminoma | IIIC | Laparotomy | BSO + TAH + OT +
LND | No | Yes | BEP (8) | No |
| 3 | 21 | Dysgerminoma | IB | Laparotomy | BSO + TAH + OT +
LND | No | Yes | BEP (3) | No |
| 4 | 22 | Dysgerminoma | IB | Laparotomy | USO + contralateral
CT + OT | Yes | No | BEP (4) | No |
| 5 | 17 | Yolk sac tumor | IB | Laparotomy | BSO + TAH + OT +
LND | No | Yes | PVB (1) | No |
| 6 | 24 | Choriocarcinoma | IC | Laparoscopy | CT + contralateral
lesion resection | Yes | No | CDF (6) | Yes |
| 7 | 17 | Dysgerminoma with
gonadoblastoma | IB | Laparoscopy | BSO | No | No | BEP (3) | No |
| 8 | 17 | Dysgerminoma with
gonadoblastoma | IB | Laparoscopy | BSO | No | No | No | No |
The median age of the patients was 18 years (range,
17–24 years). A total of 5 patients were admitted to the hospital
complaining of a pelvic mass, 1 of irregular vaginal bleeding, and
the remaining 2 patients of absence of menarche at the age of 17
years. One patient also complained of frequent urination as an
associated symptom. All the patients were nulliparous.
Diagnosis
Two patients presented with bilateral congenital
gonadal dysgenesis. The mass in the remaining 6 patients was sized
4.7–30 cm. The tumor size of the bilateral ovaries differed
significantly in the same patient, with only one side presenting
with an obviously enlarged or giant ovary, while the other ovary
was only mildly or moderately enlarged. One patient was diagnosed
with FIGO stage IIIC disease, whereas the remaining patients were
all FIGO stage IB or IC. Four patients were diagnosed with
dysgerminoma, 2 with dysgerminoma coexisting with gonadoblastoma,
and the remaining 2 with yolk sac tumor or ovarian primary
choriocarcinoma.
Treatment
Laparotomy was performed in 5 and laparoscopy in 3
patients. Four patients underwent complete staging procedures.
Fertility was spared in 2 patients (details listed in Table I). All the patients received
postoperative chemotherapy, apart from 1 patient diagnosed with
dysgerminoma coexisting with gonadoblastoma. The chemotherapy
regimens were bleomycin, etoposide and cisplatin (BEP) in 4
patients; cisplatin, vincristine and bleomycin (PVB) in 2 patients
and carboplatin, dactinomycin and 5-fluorouracil (CDF) in 1
patient. The number of cycles ranged from 1 to 8.
Follow-up
During the median follow-up of 83 months (range,
11–429 months), all the patients remained alive. Only 1 patient
with bilateral ovarian primary choriocarcinoma experienced
recurrence. The patient was a 24-year-old woman who complained of
irregular vaginal bleeding for ~1 month, with a serum β-human
chorionic gonadotropin (β-HCG) level elevated to 168.67 IU/l
(normal, <2.7 IU/l). The patient was first diagnosed with
ectopic pregnancy and a laparoscopic examination was implemented. A
5-cm cyst was found in the left ovary and a minor break was present
in the right ovary which was bleeding, and was considered to be a
corpus luteum. The left ovarian cyst was excised and the corpus
luteum-like right ovarian lesion was also stripped. On histological
examination, both lesions were proven to be primary
choriocarcinomas. Following surgery, the CDF regimen was
administered for 6 cycles: Carboplatin i.p. on day 1, followed by
dactinomycin and 5-fluorouracil i.v. on days 2–6. The β-HCG level
returned to normal, but increased again before the fifth cycle.
After the sixth cycle, a second laparotomy was performed, including
BSO, hysterectomy, omentectomy, pelvic lymph node dissection and
appendectomy. Subsequently, 1 cycle of the CDF regimen and 7 cycles
of etoposide, methotrexate, actomycin D, cyclophosphamide and
vincristine (EMA-CO) were administered. The β-HCG returned to
normal after the fourth cycle of EMA-CO and did not increase
again.
Discussion
Bilateral MOGCTs are rare, with a reported
prevalence of 4.3–6.9% among MOGCT patients. In the present study,
6.2% of our MOGCT patients exhibited bilateral involvement, which
was consistent with previously reported data. The most common
histological type in our series was dysgerminoma, which was also
consistent with the published data (5). MOGCTs coexisting with gonadoblastoma in
both ovaries are also common, whereas bilateral ovarian yok sac
tumor, immature teratoma, embryonal carcinoma and choriocarcinoma
are sporadic. The prognosis of bilateral MOGCTs is satisfactory,
with no significant difference in OS and DFS between unilateral and
bilateral tumors. This result is consistent with a previous study
investigating patients identified from the Surveillance,
Epidemiology, and End Results (SEER) program, which is the national
cancer registry of the United States; the study involved 1,529
patients affected by MOGCTs over a period of 19 years and
demonstrated that bilaterality was not an independent predictor of
survival (2).
As regards to the treatment of bilateral MOGCTs, we
should first emphasize the importance of USG evaluation prior to
surgery and careful exploration of bilateral adnexa during surgery,
prior to the excision of any tissue. It is particularly important
as a sizeable ovarian mass may occupy most of the pelvic cavity,
making it difficult to evaluate the contralateral ovary. When
bilateral MOGCT is confirmed during surgery, the treatment
modalities should be based on the histological type to a certain
extent, according to the published literature and our results.
Dysgerminoma is the female analogue of seminoma and
the most common type of MOGCT (8).
The majority (75%) of the patients are diagnosed as FIGO stage I
and the 5-year survival of the affected patients is 95% (8). Adjuvant chemotherapy is the only
independent prognostic factor for longer DFS and is considered
essential for all patients, apart from those with stage IA disease
(8).
Bilateral ovarian involvement occurs in 10–15% of
dysgerminoma patients (8). The 5-year
survival rate for bilateral dysgerminoma is reported to be 96%, and
bilaterality was not found to be an independent prognostic
predictor of survival (3). Thus,
treatment modalities should be based on those for unilateral
disease. It was suggested that, if normal ovarian tissue can be
identified, unilateral salpingo-oophorectomy plus contralateral
cystectomy or bilateral cystectomy should be taken into
consideration (9). However, if both
ovaries are almost replaced by neoplastic tissue, attempting to
preserve even a small part of the ovary is very likely to result in
residual disease. Nevertheless, advanced-stage patients with
residual lesions treated with chemotherapy had a satisfactory
prognosis (10). In an Italian study,
the authors suggested only dysgerminoma histology should be
considered for fertility-preserving surgery if residual lesions
cannot be avoided (5). Vicus et
al reported the case of a 17-year-old patient with bilateral
dysgerminoma (FIGO stage IB), who was left with a residual lesion
in an attempt to spare fertility. After receiving chemotherapy, the
patient had remained relapse-free for 5 years at the time of
publication of that case (11).
Careful surveillance, including imaging tests and measurement of
serum biomarkers, such as carbohydrate antigen-125, is emphasized.
Even if both ovaries are destroyed by the tumors, preservation of
the uterus for future in vitro fertilization-embryo transfer
(IVF-ET) using a donated egg may also be a reasonable choice. In
our series, 1 of the 4 bilateral dysgerminoma patients received
fertility-sparing surgery; her menstrual cycles remained regular
after 4 cycles of BEP regimen and no recurrence was detected during
the 102-month follow-up.
MOGCTs coexisting with gonadoblastoma account for
10% of all MOGCTs (12). The most
common complaint of the affected patients is absence of menarche.
USG examination reveals gonadal congenital dysgenesis, and
chromosome examination usually reveals the 46,XY karyotype.
Prophylactic bilateral gonadectomy is recommended due to the
increased risk of malignant transformation (13), relating to aberrant Y chromosome gene
mutation (14) and microsatellite
instability (15). We consider
laparoscopy to be safe for such patients. There is no consensus
regarding postoperative chemotherapy, as gonadoblastoma, which is a
benign neoplasm, usually overgrows other constituents. Hormone
therapy may be selected after surgery and successful pregnancy was
reported to be achieved in a 46,XY karyotype patient using IVF-ET
with a donated egg (16).
Yolk sac tumors are highly aggressive malignancies,
usually characterized by early intra-abdominal dissemination and
metastasis (17). The reported 5-year
survival rates for stage I, II, III and IV disease were 95, 75, 30
and 25%, respectively (18). In
addition to the FIGO stage, it was reported that cytoreductive
surgery significantly affected the prognosis (19). Another study also suggested that the
presence of residual tumor was a significant prognostic factor of
recurrence (17). These results all
support that optimal cytoreductive surgery is vital. The reported
rates of bilateral involvement ranged from 6 to 8.4% (17,19). For
bilateral yolk sac tumors, fertility-preserving surgery should be
performed with caution, as possible residual disease is very likely
to compromise the outcome.
Primary ovarian choriocarcinoma is rare, with an
estimated incidence of 1 in 389,000,000 women (20). Bilateral choriocarcinoma of ovarian
origin is even rarer. This tumor is highly aggressive and the
prognosis is usually poor. For bilateral disease, fertility-sparing
surgery is not recommended (20).
Postoperative chemotherapy is very important, although, compared
with gestational choriocarcinoma, primary ovarian tumors are more
chemoresistant (21). The BEP regimen
is recommended, as primary ovarian choriocarcinoma is considered to
be a germ cell tumor differentiating towards trophoblastic
structures (20). However, good
prognosis may also be achieved with the EMA-CO regimen (22). Dactinomycin and 5-fluorouracil were
reported to exhibit good efficacy when treating primary tubal
choriocarcinoma and gestational choriocarcinoma (23). In our case, fertility was spared at
first; however, although chemotherapy with dactinomycin and
5-fluorouracil was administered after surgery, the patient
experienced relapse. A second radical operation was performed, the
EMA-CO regimen was adopted thereafter and the patient was
salvaged.
Immature teratoma was not included in our series.
However, several cases of patients who were bilaterally affected by
immature teratoma and received cystectomy with chemotherapy have
been reported; they were all recurrence-free during a median
follow-up of 4.7 years (24). Other
types, such as bilateral embryonal carcinoma, should be treated
with caution, due to the extremely aggressive behavior of this
tumor, as the reported survival rate is only 39% (5).
In summary, we reviewed the data of patients
affected by bilateral MOGCTs over a period of 14 years in the
largest oncological center of eastern China and reviewed the
related literature, to discuss the issue of preserving fertility in
patients with bilateral involvement. The major limitation of this
study was the small patient sample. However, bilateral MOGCTs are
rare and the published studies are also rare, with limited sample
sizes (5). Large-scale studies are
required in the future to further investigate this issue.
Patients affected by bilateral MOGCTs have an
excellent prognosis. It was hypothesized that conservative
treatment should always be preferred, as the tumors are highly
sensitive to chemotherapy (5).
However, according to our experience and the literature data, the
selected treatment modalities should largely depend on the
histological type of the tumor. Fertility-sparing surgery may be
safe for patients affected by dysgerminoma; however, for primary
ovarian choriocarcinoma, the decision to preserve fertility may be
associated with significant risks.
Acknowledgements
This study was supported by grant no. 15140903000
from the Shanghai Science and Technology Commission (Y.L.) and
grant no. 201540224 from the Shanghai Municipal Commission of
Health and Family Planning (Y.L.).
References
|
1
|
Smith HO, Berwick M, Verschraegen CF,
Wiggins C, Lansing L, Muller CY and Qualls CR: Incidence and
survival rates for female malignant germ cell tumors. Obstet
Gynecol. 107:1075–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahdi H, Kumar S, Seward S, Semaan A,
Batchu R, Lockhart D, Tamimi H and Munkarah AR: Prognostic impact
of laterality in malignant ovarian germ cell tumors. Int J Gynecol
Cancer. 21:257–262. 2011.PubMed/NCBI
|
|
3
|
Roychoudhuri R, Putcha V and Møller H:
Cancer and laterality: A study of the five major paired organs
(UK). Cancer Causes Control. 17:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gershenson DM: Management of ovarian germ
cell tumors. J Clin Oncol. 25:2938–2943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sigismondi C, Scollo P, Ferrandina G,
Candiani M, Angioli R, Viganò R, Scarfone G and Mangili G:
Management of bilateral malignant ovarian germ cell tumors: A
MITO-9 retrospective study. Int J Gynecol Cancer. 25:203–207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fritz A, Percy C, Jack A, Shanmugaratnam
K, Sobin L, Parkin DM and Whelan S: International Classification of
Diseases for Oncology (ICD-O) (3rd). World Health Organization.
Geneva, Switzerland: 2013.
|
|
7
|
Mutch DG and Prat J.: 2014 FIGO staging
for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Husaini ALH, Soudy H, El Din Darwish A,
Ahmed M, Eltigani A, Mubarak ALM, Sabaa AA, Edesa W, L-Tweigeri AT
and Al-Badawi IA: Pure dysgerminoma of the ovary: A single
institutional experience of 65 patients. Med Oncol. 29:2944–2948.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brewer M, Gershenson DM, Herzog CE,
Mitchell MF, Silva EG and Wharton JT: Outcome and reproductive
function after chemotherapy for ovarian dysgerminoma. J Clin Oncol.
17:2670–2675. 1999.PubMed/NCBI
|
|
10
|
Dimopoulos MA, Papadopoulou M,
Andreopoulou E, Papadimitriou C, Pavlidis N, Aravantinos G,
Aspropotamitis A, Anagnostopoulos A, Fountzilas G, Michalas S and
Pectacides D: Favorable outcome of ovarian germ cell malignancies
treated with cisplatin or carboplatin-based chemotherapy: A
Hellenic Cooperative Oncology Group Study. Gynecol Oncol. 70:70–74.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vicus D, Beiner ME, Klachook S, Le LW,
Laframboise S and Mackay H: Pure dysgerminoma of the ovary 35 years
on: A single institutional experience. Gynecol Oncol. 117:23–26.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin KY, Bryant S, Miller DS, Kehoe SM,
Richardson DL and Lea JS: Malignant ovarian germ cell tumor - role
of surgical staging and gonadal dysgenesis. Gynecol Oncol.
134:84–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jonson AL, Geller MA and Dickson EL:
Gonadal dysgenesis and gynecologic cancer. Obstet Gynecol.
116(Suppl 2): S550–S552. 2010. View Article : Google Scholar
|
|
14
|
Canto P, Söderlund D, Reyes E and Méndez
JP: Mutations in the desert hedgehog (DHH) gene in patients with
46,XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab.
89:4480–4483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Funato T, Uehara S, Takahashi M, Kozawa K,
Satoh J, Sasaki T and Kaku M: Microsatellite instability in gonadal
tumors of XY pure gonadal dysgenesis patients. Int J Gynecol
Cancer. 12:192–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bianco S, Agrifoglio V, Mannino F, Cefalù
E and Cittadini E: Successful pregnancy in a pure gonadal
dysgenesis with karyotype 46,XY patient (Swyer's syndrome)
following oocyte donation and hormonal treatment. Acta Eur Fertil.
23:37–38. 1992.PubMed/NCBI
|
|
17
|
Cicin I, Saip P, Guney N, Eralp Y, Ayan I,
Kebudi R and Topuz E: Yolk sac tumours of the ovary: Evaluation of
clinicopathological features and prognostic factors. Eur J Obstet
Gynecol Reprod Biol. 146:210–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nawa A, Obata N, Kikkawa F, Kawai M,
Nagasaka T, Goto S, Nishimori K and Nakashima N: Prognostic factors
of patients with yolk sac tumors of the ovary. Am J Obstet Gynecol.
184:1182–1188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurman RJ and Norris HJ: Embryonal
carcinoma of the ovary: A clinicopathologic entity distinct from
endodermal sinus tumor resembling embryonal carcinoma of the adult
testis. Cancer. 38:2420–2433. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heo EJ, Choi CH, Park JM, Lee JW, Bae DS
and Kim BG: Primary ovarian choriocarcinoma mimicking ectopic
pregnancy. Obstet Gynecol Sci. 57:330–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koo HL, Choi J, Kim KR and Kim JH: Pure
non-gestational choriocarcinoma of the ovary diagnosed by DNA
polymorphism analysis. Pathol Int. 56:613–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozturk E, Ugur MG, Cebesoy FB, Aydin A,
Sever T and Balat O: Good prognosis for primary ovarian pure
nongestational choriocarcinoma using the EMA/CO regime. Eur J
Gynaecol Oncol. 31:123–125. 2010.PubMed/NCBI
|
|
23
|
Wan J, Li XM and Gu J: Primary
choriocarcinoma of the fallopian tube: A case report and literature
review. Eur J Gynaecol Oncol. 35:604–607. 2014.
|
|
24
|
Beiner ME, Gotlieb WH, Korach Y, Shrim A,
Stockheim D, Segal Y, Fridman E and Ben-Baruch G: Cystectomy for
immature teratoma of the ovary. Gynecol Oncol. 93:381–384. 2004.
View Article : Google Scholar : PubMed/NCBI
|