Introduction
Peritoneal metastasis (PM) is the most common
metastatic pattern in gastric cancer (GC) and has an extremely poor
prognosis (1,2), with a median survival of 3–6 months
without treatment (3). PM may lead to
refractory ascites, intestinal obstruction, cachexia and eventually
death. Thus far, PM is considered as stage IV disease, and
treatment is non-curative.
There is currently no established consensus to
direct treatment for GC patients with positive peritoneal cytology.
Intraperitoneal chemotherapy (IPC) has been proposed as a treatment
option, which may improve survival in GC patients patients with PM
(4–8).
IPC possesses a theoretical advantage over the systemic route by
delivering high drug concentrations directly to the peritoneal
cavity, with reduced systemic toxicity (9–11). In
addition, high drug concentrations are achieved in the portal vein
(12). This may be important, as the
liver is a common metastatic site (13). Recently, hyperthermia has been
developed as an anticancer therapy and has been shown to exert a
direct cytotoxic effect on tumor cells in the peritoneal cavity in
conjunction with certain chemotherapeutic agents (14). Zhao et al reported that
whole-body hyperthermia combined with hyperthermic intraperitoneal
chemotherapy (HIPEC) is an effective treatment for patients with
advanced gastric malignancies (15).
A meta-analysis by Sun et al demonstrated that HIPEC may
improve the overall survival (OS) rate in patients who undergo
resection for advanced GC, and help to prevent peritoneal local
recurrence among GC patients with serosal invasion (16). HIPEC has also been suggested to be
useful in treating advanced peritoneal metastatic cancer, and
significantly prolonged survival following tumor resection
(17,18).
The aim of this study was to evaluate the efficacy
and safety of HIPEC in advanced GC patients with peritoneal
dissemination by retrospective analysis.
Patients and methods
Patients
The clinical records of 54 patients who were
primarily diagnosed as GC with PM at the Zhejiang Cancer Hospital
(Hangzhou, China) from 2008-1-1 through to 2014-12-31 were
retrospectively analyzed (Table I).
Patients eligible for this study had histologically confirmed GC
with PM, which means positive peritoneal cytology. The patients had
been treated with mono- or combination chemotherapy, which was
found to be effective in GC, including S-1, S-1 + cisplatin (SP),
S-1 + oxaliplatin (SOX), capecitabine + oxaliplatin (XELOX),
capecitabine + docetaxel (XT), oxaliplatin + 5-fluorouracil +
folinic acid (FOLFOX), docetaxel + cisplatin (DP), Doxetaxel + S1
(DS), Doxetaxel + Xeloda (capecitabine) (DX) and irinotecan
(CPT-11). Finally, a total of 33 patients had complete survival
data, including 12 in the HIPEC+ and 21 in the HIPEC- group.
 | Table I.Baseline characteristics of HIPEC+ and
HIPEC- patients |
Table I.
Baseline characteristics of HIPEC+ and
HIPEC- patients
|
| HIPEC+ group | HIPEC- group |
|---|
|
|
|
|
|---|
| Characteristics | All (n=23) | Complete survival
data (n=12) | All (n=31) | Complete survival
data (n=21) |
|---|
| Gender, n |
|
|
|
|
| Male | 7 | 5 | 11 | 7 |
|
Female | 16 | 7 | 20 | 14 |
| Age, years |
|
|
|
|
|
Median | 43 | 44.5 | 53 | 52 |
|
Range | 18–68 | 31–68 | 24–77 | 24–66 |
| Primary site, n |
|
|
|
|
|
Esophagogastric junction | 1 | 1 | 1 | 0 |
| Gastric
body | 9 | 5 | 14 | 10 |
| Gastric
antrum | 10 | 5 | 9 | 6 |
| Diffuse
gastric lesions | 3 | 1 | 3 | 2 |
|
Unkown | 0 | 0 | 4 | 3 |
| Pathology, n |
|
|
|
|
|
Well-differentiated | 0 | 0 | 0 | 0 |
|
Moderately differentiated | 0 | 0 | 3 | 2 |
| Poorly
differentiated | 18 | 8 | 27 | 18 |
|
Differentiation unknown | 5 | 4 | 1 | 1 |
| Radical surgery,
n |
|
|
|
|
| Yes | 4 | 4 | 8 | 5 |
| No | 19 | 8 | 23 | 16 |
The present study was approved by the Ethics
Committee and Institutional Review Board of the Zhejiang Cancer
Hospital and was conducted in compliance with the ethical
principles of the Declaration of Helsinki.
Treatment
Among the 54 patients, 23 received systemic
chemotherapy combined with HIPEC (HIPEC+ group) and 31 patients
received systemic chemotherapy alone (HIPEC- group). The systemic
chemotherapy was a mono- or combination therapy, which was found to
be effective in GC, including S1, SP, SOX, XELOX, XT, FOLFOX, DP,
DS, DX and CPT-11. In the HIPEC+ group, the patients were
administered cisplatin (CDDP, 50 mg/m2) through
intraperitoneal perfusion during chemotherapy and then abdominal
hyperthermia was applied in vitro. The doses of chemotherapy
and their adjustments were made according to each patient's
status.
Adverse effects
Toxicity was measured using the National Cancer
Institute Common Toxicity Criteria version 2.0 toxicity scales
(19). Grade 3–4 toxicity was
recorded according to the medical records.
Assessment and statistics
Response was evaluated every two cycles of treatment
using the Response Evaluation Criteria in Solid Tumors (20). The patients were divided into 4
categories according to the changes in ascites, including
disappear, decrease, stable and increase. Survival time was
analyzed with the Kaplan-Meier method using the SPSS software,
version 15.0 (SPSS, Inc., Chicago, IL, USA).
Results
Efficacy
The patients were divided into 4 categories
according to the changes of ascites, namely disappear, decrease,
stable and increase. In the 54 patients, the disappear + decrease
rate in the HIPEC+ group was 82.60%, which was superior to that in
the HIPEC- group (54.80%; P=0.043). The disappear + decrease +
stable rate was 95.70% in the HIPEC+ group and 74.20% in the HIPEC-
group (P=0.062). The disease control rate was 65.22% in the HIPEC+
group and 58.06% in the HIPEC- group (P=0.594). Therefore, HIPEC
may effectively control ascites in advanced GC.
Of the 33 patients with complete survival data
available, including 12 from the HIPEC+ and 21 from the HIPEC-
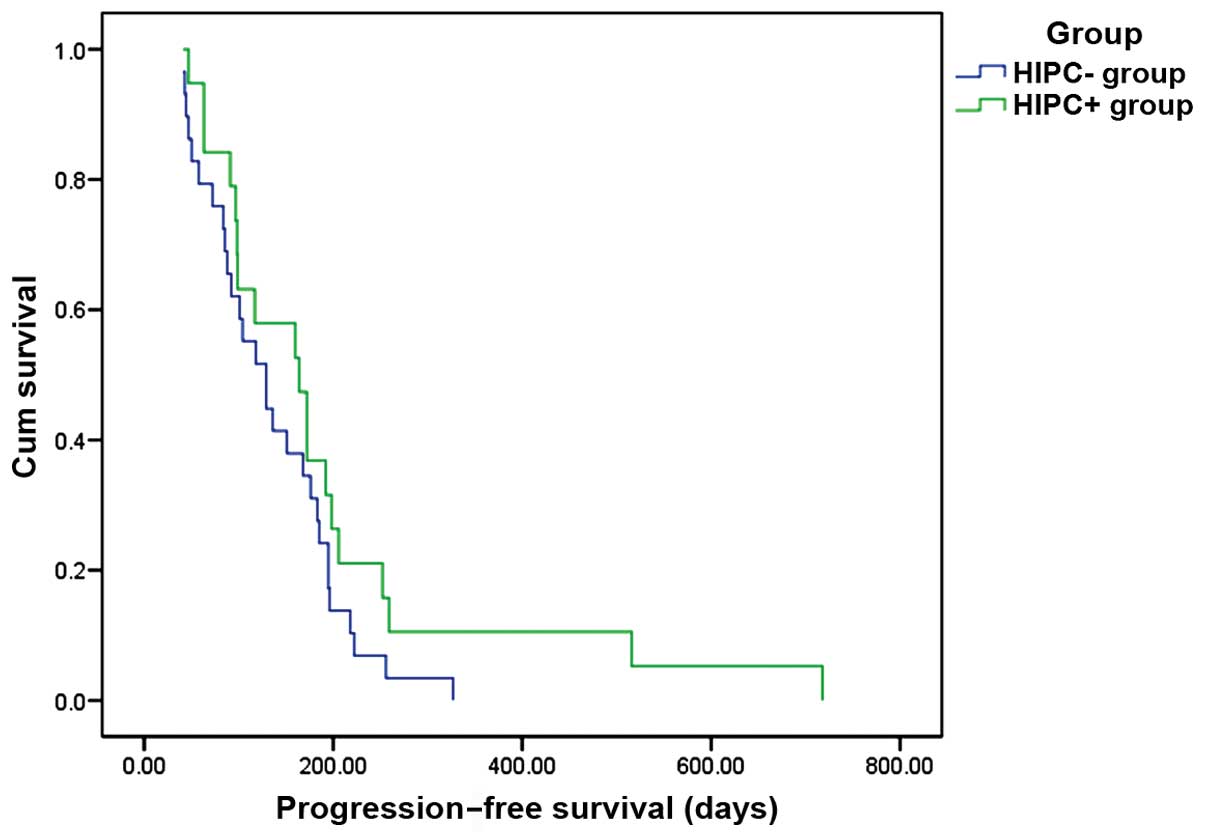
group, further analysis revealed that the median progression-free
survival (PFS) was 164 days in the HIPEC+ group and 129 days in the
HIPEC- group (P=0.158; Fig. 1). The
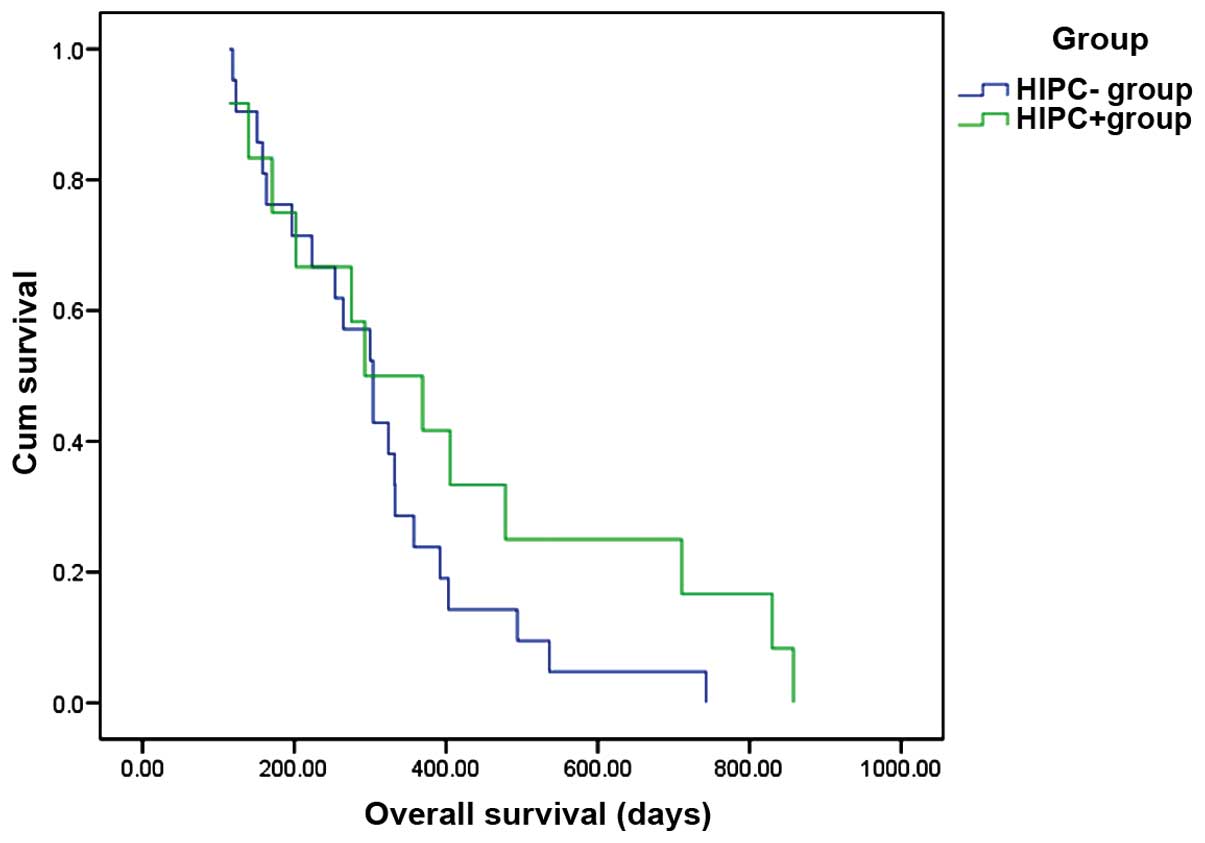
median OS was 494 days in the HIPEC+ and 223 days in the HIPEC-
group (P=0.178), and the 1-year survival rate was 41.7 and 23.8%,
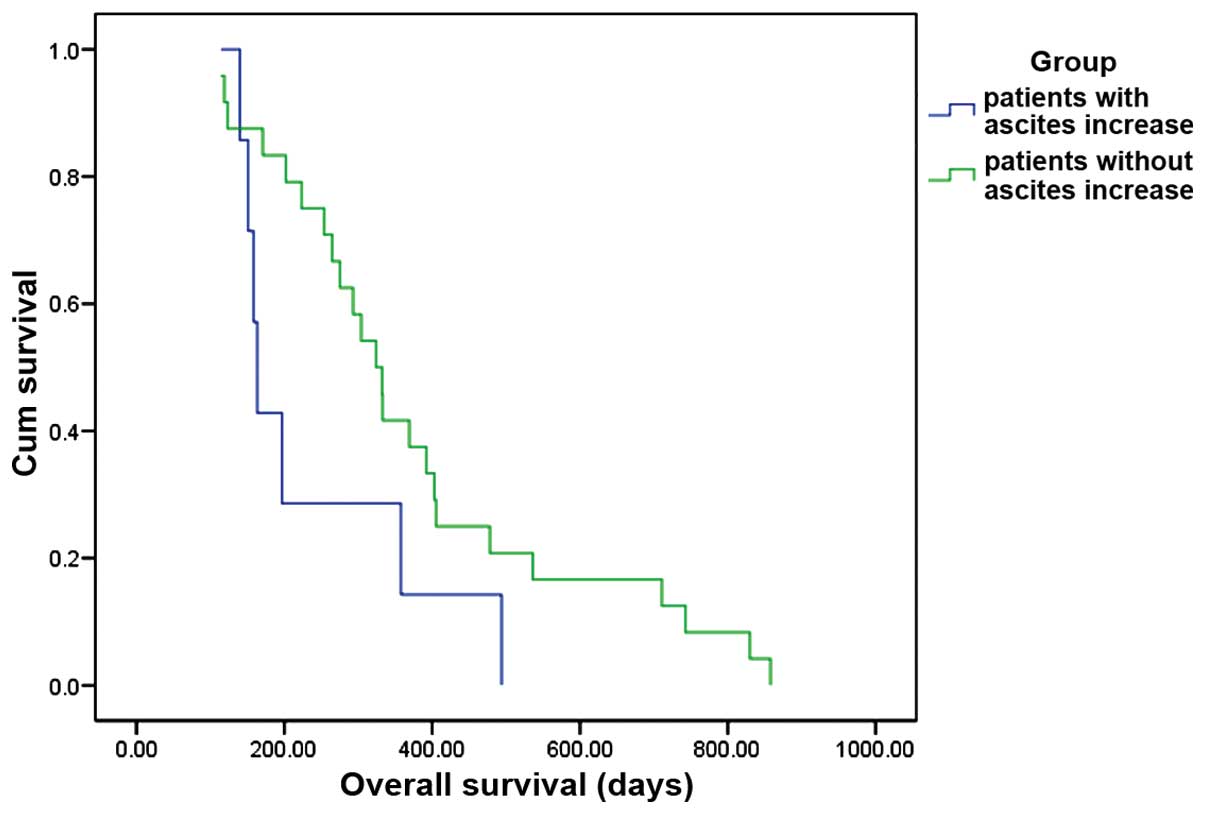
respectively (P<0.001; Fig. 2). In
patients with ascites disappear + decrease + stable, the OS
appeared to be better compared with that in patients with ascites
increase, but the difference was not statistically significant
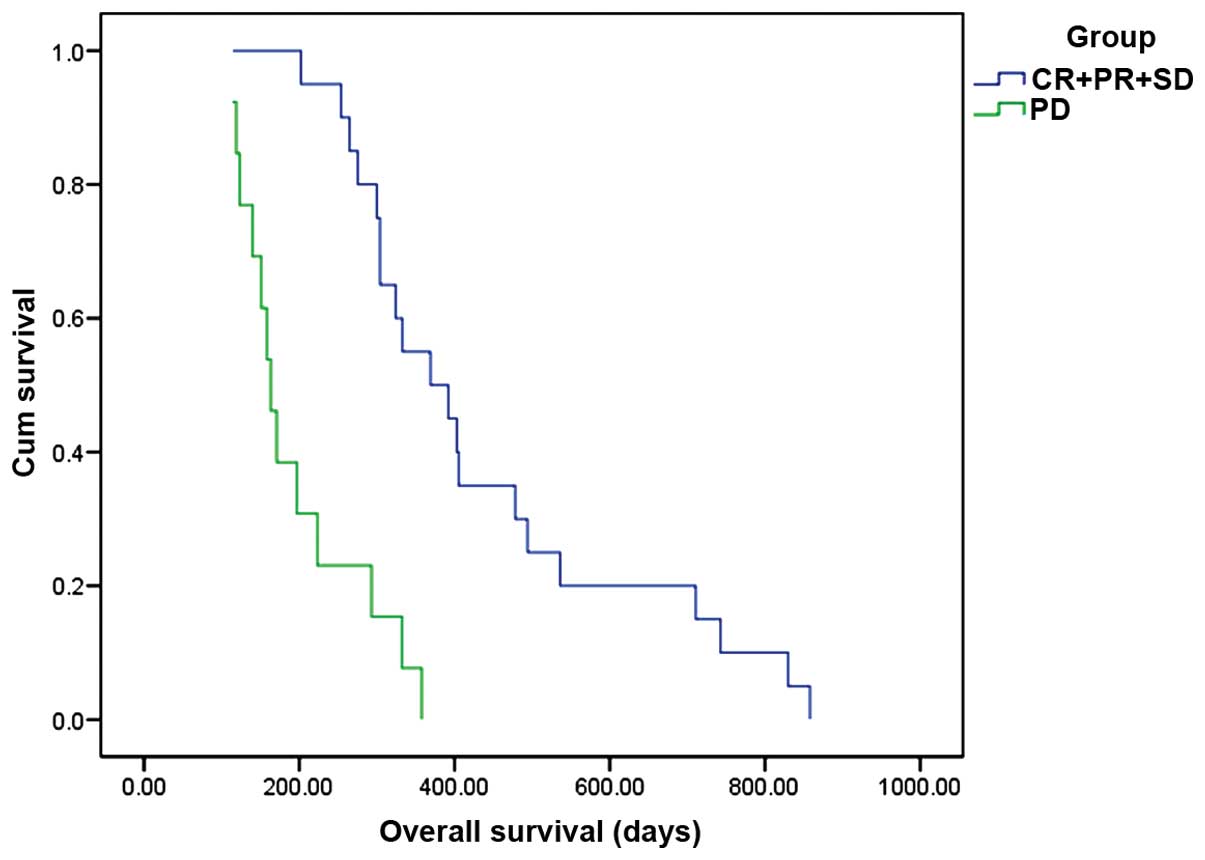
(P=0.08; Fig. 3). Patients with
controlled disease [complete response (CR) + partial response (PR)
+ stable disease (SD)], may have a better OS compared with patients
with progressive disease (P<0.001; Fig. 4). Other factors, such as gender, age,
primary tumor site, pathology, and radical surgery, exhibited no
obvious correlation with OS (Table
II).
 | Table II.Correlation analysis between baseline
characteristics and overall survival (n=33) |
Table II.
Correlation analysis between baseline
characteristics and overall survival (n=33)
| Variables | P-value |
|---|
| Gender | 0.102 |
| Age | 0.967 |
| Primary site | 0.731 |
| Pathology | 0.910 |
| Radical
operation | 0.178 |
Toxicity
All 54 patients were assessed for treatment safety,
and no grade 3 or 4 adverse events or treatment-related deaths were
recorded.
Discussion
HIPEC has been proven to be effective in the
treatment of PM in ovarian, appendiceal and colorectal cancer
(21–24), but its role in GC with PM has not been
established. The present study retrospectively investigated GC
patients with PM from 2008-1-1 through to 2014-12-31 at the
Zhejiang Cancer Hospital. The results demonstrated that the
disappear + decrease rate in the HIPEC+ group was better compared
with that in the HIPEC- group, which means that HIPEC may
effectively control ascites and significantly improve the quality
of life in GC patients with PM. This may be a major step in the
fight against cancer.
In patients with complete survival data, the
survival curves of the two groups were separated in Kaplan-Meier
estimates of PFS and OS: The 1-year survival rate of HIPEC+ group
was significantly higher compared with that of the HIPEC- group.
Recent studies on GC patients with PM treated with cytoreductive
surgery (CRS) and HIPEC reported 1-year OS rates ranging from 35.5
to 50% (Table III) (25–32). Our
results are similar, but considered encouraging, due to the
avoidance of surgical trauma. However, the difference in PFS and OS
between the two groups was not statistically significant. This may
be attributed to the small number of cases, which suggests that
further studies with larger samples are required to confirm our
data.
 | Table III.Clinical results of GC patients with
PM treated with CRS and HIPEC. |
Table III.
Clinical results of GC patients with
PM treated with CRS and HIPEC.
|
|
|
|
|
| Temperature
(°C) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Authors
(Refs.) | Publication
year | Country | No. of
patients | Drug regimen | Inflow | Outflow | Median survival
(months) | 1-year survival
rate (%) |
|---|
| Fujimoto et
al (25) | 1997 | Japan | 48 | MMC | 44.5–45.0 | 43–44 | | 54.0 |
| Beaujard et
al (26) | 2000 | France | 42 | MMC | 46–49 | 36–43 | | 48.0 |
| Mussa et al
(27) | 2001 | Italy | 7 | MMC | | 42–43 | | 68.0 |
| Yonemura et
al (28) | 2005 | Japan | 107 | MMC, CDDP, ETP |
| 42–43 | 11.5 | 35.5 |
| Glehen et al
(29) | 2010 | France | 159 | MMC, CDDP or OXIRI,
5-FU |
| 40–43 | 9.2 | 43.0 |
| Yang et al
(30) | 2010 | China | 21 | Hydroxycamptochecin
or CDDP, MMC | | 43 | | 50.0 |
| Yang et al
(31) | 2011 | China | 34 | MMC, CDDP | | 43 | 11.0 | 41.2 |
| Magge et al
(32) | 2013 | USA | 23 | MMC, CDDP | | 42 | 9.5 | 50.0 |
Further analysis in our study demonstrated that in
patients with ascites disappear + decrease + similar, the OS
appeared to be better compared with that in patients with ascites
increase; the survival curves of the two groups were separated, but
the difference was not statistically significant. In addition,
patients with controlled disease (CR + PR + SD), may have a better
OS compared with patients with progressive disease (P<0.001).
These results indicate that short-term remission of the disease,
including control of ascites, is associated with good prognosis.
Further research on the association between short-term remission
and the prognosis of advanced GC is planned in the near future.
Toxicity is also a major concern. As HIPEC has the
advantage of increasing the concentration of chemotherapeutic
agents locally administered into the peritoneum with fewer systemic
side effects compared with systemic chemotherapy, it may be
hypothesized that the combination of heat and drug toxicity may
lead to more complications, such as anastomotic leakage,
intra-abdominal abscess, wound infection, pulmonary edema and adult
respiratory distress syndrome (25).
In this study, none of the abovementioned serious adverse effects
were observed, and no other serious unacceptable adverse effects or
treatment-related deaths were reported.
In summary, this study demonstrated the
effectiveness and safety of HIPEC combined with systemic
chemotherapy for advanced GC with peritoneal dissemination;
however, large multicenter prospective randomized controlled
studies and pharmacokinetic analysis are required to confirm our
findings.
Acknowledgements
The present study was supported by the General
Research Program of Medical Health in Zhejiang Province (grant nos.
2011KYA032, 2014KYB039 and 2016KYB036), the Scientific Research
Fund Project of Integrated Chinese and Western Medicine Institute
in Zhejiang province (grant no. 2014LYK021), the Science and
Technology in Zhejiang Province Chinese Medicine Program (grant
nos. 2012ZA101 and 2016ZA038), the Hangzhou City Science and
Technology Project Planning Guide (Social Development) (grant no.
20130733Q15) and the Hangzhou City Health Science and Technology
Project (grant no. 2013A43).
References
|
1
|
Brigand C, Arvieux C, Gilly FN and Glehen
O: Treatment of peritoneal carcinomatosis in gastric cancers. Dig
Dis. 22:366–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 annual report
of the JGCA Nationwide Registry. Gastric Cancer. 14:301–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yonemura Y, Endou Y, Sasaki T, Hirano M,
Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M and Li Y:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodera Y, Ito Y, Ito S, Ohashi N,
Mochizuki Y, Yamamura Y, Koike M, Fujiwara M, Nakanishi H and Nakao
A: Intraperitoneal paclitaxel: A possible impact of regional
delivery for prevention of peritoneal carcinomatosis in patients
with gastric carcinoma. Hepatogastroenterology. 54:960–963.
2007.PubMed/NCBI
|
|
5
|
Ishigami H, Kitayama J, Otani K, Kamei T,
Soma D, Miyato H, Yamashita H, Hidemura A, Kaisaki S and Nagawa H:
Phase I pharmacokinetic study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer. Oncology. 76:311–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishigami H, Kitayama J, Kaisaki S,
Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H
and Nagawa H: Phase II study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer with peritoneal metastasis. Ann Oncol. 21:67–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishigami H, Kitayama J, Kaisaki S,
Yamaguchi H, Yamashita H, Emoto S and Nagawa H: Phase I study of
biweekly intravenous paclitaxel plus intraperitoneal cisplatin and
paclitaxel for gastric cancer with peritoneal metastasis. Oncology.
79:269–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matharu G, Tucker O and Alderson D:
Systematic review of intraperitoneal chemotherapy for gastric
cancer. Br J Surg. 98:1225–1235. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yonemura Y, Ninomiya I, Kaji M, Sugiyama
K, Fujimura T, Sawa T, Katayama K, Tanaka S, Hirono Y, Miwa K, et
al: Prophylaxis with intraoperative chemohyperthermia against
peritoneal recurrence of serosal invasion-positive gastric cancer.
World J Surg. 19:450–454; discussion 455. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dedrick RL: Theoretical and experimental
bases of intraperitoneal chemotherapy. Semin Oncol. 12(3 Suppl 4):
S1–S6. 1985.
|
|
11
|
Shimada T, Nomura M, Yokogawa K, Endo Y,
Sasaki T, Miyamoto K and Yonemura Y: Pharmacokinetic advantage of
intraperitoneal injection of docetaxel in the treatment for
peritoneal dissemination of cancer in mice. J Pharm Pharmacol.
57:177–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Speyer JL, Sugarbaker PH, Collins JM,
Dedrick RL, Klecker RW Jr and Myers CE: Portal levels and hepatic
clearance of 5-fluorouracil after intraperitoneal administration in
humans. Cancer Res. 41:1916–1922. 1981.PubMed/NCBI
|
|
13
|
Landry J, Tepper JE, Wood WC, Moulton EO,
Koerner F and Sullinger J: Patterns of failure following curative
resection of gastric carcinoma. Int J Radiat Oncol Biol Phys.
19:1357–1362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiu MH and Fortner JG: Intraperitoneal
hyperthermic treatment of implanted peritoneal cancer in rats.
Cancer Res. 40:4081–4084. 1980.PubMed/NCBI
|
|
15
|
Zhao C, Dai C and Chen X: Whole-body
hyperthermia combined with hyperthermic intraperitoneal
chemotherapy for the treatment of stage IV advanced gastric cancer.
Int J Hyperthermia. 28:735–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Song Y, Wang Z, Gao P, Chen X, Xu
Y, Liang J and Xu H: Benefits of hyperthermic intraperitoneal
chemotherapy for patients with serosal invasion in gastric cancer:
A meta-analysis of the randomized controlled trials. BMC Cancer.
12:5262012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esquivel J, Elias D, Baratti D, Kusamura S
and Deraco M: Consensus statement on the loco regional treatment of
colorectal cancer with peritoneal dissemination. J Surg Oncol.
98:263–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Portilla Gómez A: Peritoneal
carcinomatosis. Ten years of applying the new combined triple
therapy. Personal experience. Cir Esp. 82:346–351. 2007.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimizu T and Saijo N: Common toxicity
criteria: version 2.0, an improved reference for grading the
adverse reaction of cancer treatment. Nihon Rinsho. 61:937–942.
2003.PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alberts DS, Markman M, Armstrong D,
Rothenberg ML, Muggia F and Howell SB: Intraperitoneal therapy for
stage III ovarian cancer: A therapy whose time has come! J Clin
Oncol. 20:3944–3946. 2002.PubMed/NCBI
|
|
22
|
Jaaback K, Johnson N and Lawrie TA:
Intraperitoneal chemotherapy for the initial management of primary
epithelial ovarian cancer. Cochrane Database Syst Rev CD005340.
2011. View Article : Google Scholar
|
|
23
|
Verwaal VJ, van Ruth S, de Bree E, van
Slooten GW, van Tinteren H, Boot H and Zoetmulder FA: Randomized
trial of cytoreduction and hyperthermic intraperitoneal
chemotherapy versus systemic chemotherapy and palliative surgery in
patients with peritoneal carcinomatosis of colorectal cancer. J
Clin Oncol. 21:3737–3743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugarbaker PH: New standard of care for
appendiceal epithelial neoplasms and pseudomyxoma peritonei
syndrome? Lancet Oncol. 7:69–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujimoto S, Takahashi M, Mutou T,
Kobayashi K, Toyosawa T, Isawa E, Sumida M and Ohkubo H: Improved
mortality rate of gastric carcinoma patients with peritoneal
carcinomatosis treated with intraperitoneal hyperthermic
chemoperfusion combined with surgery. Cancer. 79:884–891. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beaujard AC, Glehen O, Caillot JL,
Francois Y, Bienvenu J, Panteix G, Garbit F, Grandclément E, Vignal
J and Gilly FN: Intraperitoneal chemohyperthermia with mitomycin C
for digestive tract cancer patients with peritoneal carcinomatosis.
Cancer. 88:2512–2519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mussa A, Sandrucci S and Zanon C:
Intraoperative chemohyperthermia for advanced gastric cancer: A new
procedure with closed abdomen and previously constructed
anastomosis. Tumori. 87(Suppl): S18–S20. 2001.PubMed/NCBI
|
|
28
|
Yonemura Y, Kawamura T, Bandou E,
Takahashi S, Sawa T and Matsuki N: Treatment of peritoneal
dissemination from gastric cancer by peritonectomy and
chemohyperthermic peritoneal perfusion. Br J Surg. 92:370–375.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glehen O, Gilly FN, Arvieux C, Cotte E,
Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F and Elias
D: Association Française de Chirurgie: Peritoneal carcinomatosis
from gastric cancer: A multi-institutional study of 159 patients
treated by cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy. Ann Surg Oncol. 17:2370–2377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang XJ, Li Y and Yonemura Y:
Cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy to treat gastric cancer with ascites and/or peritoneal
carcinomatosis: Results from a Chinese center. J Surg Oncol.
101:457–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL,
Cheng FL, Zhou YF, Xiong B, Yonemura Y and Li Y: Cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy improves
survival of patients with peritoneal carcinomatosis from gastric
cancer: Final results of a phase III randomized clinical trial. Ann
Surg Oncol. 18:1575–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Magge D, Zenati M, Mavanur A, Winer J,
Ramalingam L, Jones H, Zureikat A, Holtzman M, Lee K, Ahrendt S, et
al: Aggressive locoregional surgical therapy for gastric peritoneal
carcinomatosis. Ann Surg Oncol. 21:1448–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|