Introduction
Breast cancer is the most commonly diagnosed cancer
in women. According to the Surveillance, Epidemiology and End
Results database, it is the third most common cause of
cancer-associated mortality in women (1). Although certain preventive approaches or
early screening programs may reduce the risk for breast cancer, the
majority of cases cannot be eliminated, particularly in developing
countries where breast cancer is diagnosed in late stages. Early
detection is therefore vital to improve the outcome and survival
and is the cornerstone for the management of breast cancer
patients. Tumor markers have been widely used for assessment of
treatment responses, early diagnosis of recurrence and prognosis.
In breast cancer, the most generally used serum tumor marker is
cancer antigen 15–3 (CA 15–3); however, its sensitivity and
specificity are inadequate (2,3). Numerous
serum markers have been studied, however, none have been
implemented for routine clinical practice (4).
Human epididymal protein 4 (HE4) is a secretory
protein initially identified in epithelial cells of the human
epididymis (5). Expression of HE4 has
been demonstrated in numerous types of normal human tissues,
particularly in the epithelium of the respiratory and genitourinary
tracts of men and women, and increased HE4 expression has been
demonstrated in a range of malignant neoplasms, particularly those
of gynecological, pulmonary and gastrointestinal origin (6–9).
As recently reported, HE4 is also expressed in
ductal carcinoma of the breast tissue (6); however, the serum expression levels and
their diagnostic and prognostic potential in breast cancer remain
to be elucidated. The aim of the present study was to examine the
association between the serum expression levels of HE4 and the
clinicopathological variables, and to assess the potential use of
circulating HE4 for the diagnosis of breast cancer.
Materials and methods
The present study was approved by the ethics
committee of Bakırköy Education and Research Hospital (Istanbul,
Turkey), and written informed consent was provided prior to the
assessment. This prospective clinical trial was performed at the
Department of Medical Oncology (Bakırköy Education and Research
Hospital (Istanbul, Turkey). The patient cohort consisted of 63
women: 36 with breast cancer, 11 with ovarian cancer, and 16 age-
and body mass index-matched healthy volunteers. Breast and ovarian
cancer patients who had undergone chemotherapy or radiation therapy
following surgery and patients with other types of cancer were
excluded.
The characteristics of the breast cancer patients
with regards to age, menopausal status, histopathological type,
tumor size, tumor lymph node metastasis and tumor grade, as well as
estrogen receptor (ER), progesterone receptor (PR), human epidermal
growth factor-2 (HER2) status, lymphovascular invasion, perineural
invasion status and stage were collected for data analysis.
The pathological tumor stage was defined according
to the seventh edition of the tumor-nodes-metastasis Classification
of Malignant Tumours of the Union for International Cancer Control
(10). Tumor differentiation was
defined according to the World Health Organization Classification
of Tumours of the Breast, fourth edition (11). Tumors were classified as >2 cm, 2–5
cm or >5 cm; tumor size and lymph node metastasis status were
evaluated separately. ER, PR and HER2 data were obtained from the
pathology records of the patients.
Blood sample collection
Venous blood samples were collected in tubes from
the antecubital vein following an overnight fast. The tubes were
centrifuged at 2,000 × g for 10 min to separate the plasma and the
serum. The plasma and the serum samples were kept at −80°C until
analysis of the HE4 levels.
Measurement of HE4
HE4 was measured using an enzyme-linked
immunosorbent assay based on the biotin double antibody sandwich
technology (One Step RT-PCR kit) according to the manufacturer's
protocol (Shanghai Yehua Biological Technology, Shanghai, China).
The range of reference values was 0.5–150 pmol/l.
Other variables
CA 125, carcinoembryonic antigen (CEA) and CA 15–3
were measured in the serum samples. The complete blood count (CBC)
was determined from whole blood samples containing ethylenediamine
tetraacetate.
The CBC was determined in a Coulter LH 750
autoanalyzer (Beckman Coulter, Brea, CA, USA). CA 125, CEA and CA
15–3 were determined using a Beckman Coulter AU5800 chemistry
autoanalyzer and DXI 800 systems using commercial kits (all from
Beckman Coulter) with a one-step dual monoclonal antibody assay
method. Ranges of reference values were as follows: CA 125, 0–11.1
U/ml; CA 15–3, 0–31.3 U/ml; and CEA, 0–3.0 ng/ml.
Statistical analysis
Statistical analysis was performed using the Number
Cruncher Statistical System (NCSS) 2007 and Power Analysis and
Sample Size 2008 statistical software (NCSS, LLC, Kaysville, Utah,
USA). Descriptive statistical methods were used for the evaluation
of study variables. For comparison of variables with a normal
distribution, the Kruskal Wallis test was used, and the
Mann-Whitney U test was used for comparison of variables with a
non-Gaussian distribution. Spearman's rho correlation analysis was
used to assess the association between HE4 and CA 15–3, which did
not show conformity with a normal distribution. Receiver operating
characteristic (ROC) analysis was used for the determination of the
aptness of HE4 for clinical differentiation between the patient and
the control groups. When the area under the curve (AUC) was found
to be significant, the cutoff values were determined and
sensitivity and specificity for that particular cutoff point were
calculated. The 95% confidence interval (CI) was evaluated for the
results. P<0.05 was considered to indicate a statistically
significant difference.
Results
Serum levels of HE4
The serum levels of HE4 were determined in 36 breast
cancer patients, 11 ovarian cancer patients and 16 healthy
volunteers. The association between the clinicopathological
characteristics of breast cancer and serum levels of HE4 was
investigated.
Associations bewteen HE4 and
clinicopathological characteristics
No significant differences were observed between the
mean age of the breast cancer patients, ovarian cancer patients and
healthy volunteers (61.39±12.99, 60.0±15.57 and 58.19±10.88 years,
respectively, P=0.739).
There was a significant difference in the median
serum levels of HE4 in breast cancer patients, ovarian cancer
patients and healthy volunteers (14.63, 16.47 and 11.52 pmol/l,
respectively; P=0.013). No significant differences between the
breast cancer and ovarian cancer patient groups was observed, the
median serum levels of HE4 in these groups were significantly
higher than those in the healthy volunteer group (P=0.006 and
P=0.017, respectively) (Table I).
 | Table I.Comparison of HE4 levels in patients
with breast cancer and the healthy controls. |
Table I.
Comparison of HE4 levels in patients
with breast cancer and the healthy controls.
|
|
| HE4 level,
pmol/l |
|
|---|
|
|
|
|
|
|---|
| Group | Total, n | Mean ± standard
deviation | Min-max (median) | P-value |
|---|
| Control | 16 | 11.94±2.19 |
8.91–16.52 (11.52) | 0.013a |
| Breast cancer | 36 |
26.83±26.19 |
10.05–107.37 (14.63) | 0.006b |
| Ovarian cancer | 11 |
21.12±14.05 | 10.20–50.05
(16.47) | 0.017b |
No significant associations between the serum levels
of HE4 with comorbidity, menopausal status, hormone receptor
status, HER-2 status, lymphovascular invasion, perineural invasion
or metastases were identified in breast cancer patients (P≥0.05).
In addition, no differences between the median serum levels of HE4
in breast cancer patients and the tumor grade, histopathological
subgroup, lymph node metastases and stage (P≥0.05) (Table II).
 | Table II.Comparison of clinicopathological
features and the serum HE4 levels in breast cancer patients. |
Table II.
Comparison of clinicopathological
features and the serum HE4 levels in breast cancer patients.
|
|
| HE4 level,
pmol/l |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Total, % | Mean ± standard
deviation | Median | P-value |
|---|
| Menopausal
status |
|
|
|
|
| Post | 72.2 | 24.25±26.12 | 12.54 | 0.241 |
| Pre | 27.8 | 27.82±26.67 | 16.02 |
|
| Comorbidity |
|
|
|
|
| (−) | 58.3 | 31.56±26.44 | 16.02 | 0.849 |
| (+) | 41.7 | 23.45±26.12 | 13.88 |
|
| Estrogen
receptor |
|
|
|
|
| (−) | 65.7 | 31.15±28.89 | 19.20 | 0.482 |
| (+) |
| 25.28±25.48 | 13.79 |
|
| Progesterone
receptor |
|
|
|
|
| (−) | 57.1 | 27.05±21.83 | 18.16 | 0.521 |
| (+) |
| 27.48±29.97 | 13.56 |
|
| Triple negative |
|
|
|
|
| (−) | 25.7 | 28.25±29.07 | 13.83 | 0.697 |
| (+) |
| 24.54±17.75 | 18.16 |
|
| HER2 |
|
|
|
|
| (−) | 20.0 | 26.17±26.04 | 15.58 | 0.672 |
| (+) |
| 31.79±29.58 | 12.14 |
|
| Lymphovascular
invasion |
|
|
|
|
| (−) | 40.0 | 26.55±23.27 | 16.02 | 0.538 |
| (+) |
| 28.41±31.48 | 12.62 |
|
| Perineural
invasion |
|
|
|
|
| (−) | 37.5 | 26.98±25.18 | 14.63 | 0.833 |
| (+) |
| 31.46±31.38 | 16.80 |
|
| Metastasis |
|
|
|
|
| (−) | 13.9 | 25.20±23.88 | 13.79 | 0.191 |
| (+) |
| 36.93±39.74 | 20.25 |
|
| Histopathological
type |
|
|
|
|
| Invasive
ductal carcinoma | 75.0 | 28.20±24.59 | 16.27 | 0.113 |
|
Other |
| 23.92±33.78 | 11.84 |
|
| Grade |
|
|
|
|
| 1 | 3.2 | 13.88 | 13.88 | 0.777 |
| 2 | 64.5 | 29.52±30.05 | 14.58 |
|
| 3 | 32.3 | 25.89±22.10 | 19.21 |
|
| Depth of
invasion |
|
|
|
|
| 1 | 13.9 | 22.41±15.76 | 15.38 | 0.655 |
| 2 | 61.1 | 26.49±26.16 | 13.83 |
|
| 3 |
8.3 | 13.62±3.85 | 12.79 |
|
| 4 | 16.7 | 38.38±37.93 | 21.00 |
|
| Lymph node
metastasis |
|
|
|
|
| 0 | 61.1 | 27.30±25.04 | 15.90 | 0.197 |
| 1 | 11.1 | 11.51±1.26 | 11.60 |
|
| 2 | 19.4 | 26.15±22.62 | 12.14 |
|
| 3 |
8.3 | 45.36±53.81 | 17.82 |
|
| Stage |
|
|
|
|
| 1 | 11.1 | 25.50±16.35 | 19.86 | 0.176 |
| 2 | 52.8 | 26.14±26.63 | 13.88 |
|
| 3 | 22.2 | 22.82±22.27 | 10.90 |
|
| 4 | 13.9 | 36.93±39.74 | 20.25 |
|
Breast cancer patients with high serum levels of CA
15–3 had a significantly higher serum level of HE4 compared to
those with low CA 15–3 levels (56.97±42.93 vs. 22.17±18.14 pmol/l,
respectively; P=0.031). However, no significant difference was
observed between the median serum level of HE4 in patient groups
with low and high serum levels of CEA and CA 125. Furthermore, no
association between the serum levels of HE4 and leukocytosis,
anemia and thrombocytosis was identified (P≥0.05) (Table III).
 | Table III.Association between HE4 levels and
blood parameters or cancer markers in breast cancer. |
Table III.
Association between HE4 levels and
blood parameters or cancer markers in breast cancer.
|
|
| HE4 level,
pmol/l |
|
|---|
|
|
|
|
|
|---|
| Variable | Total, % | Mean ± standard
deviation | Median | P-value |
|---|
| Leukocytosis |
|
|
|
|
|
(−) | 20.0 |
26.57±24.76 | 13.83 | 0.856 |
|
(+) |
|
28.60±35.14 | 15.78 |
|
| Anemia |
|
|
|
|
|
(−) | 38.2 |
26.77±27.20 | 13.24 | 0.462 |
|
(+) |
|
27.29±27.68 | 16.27 |
|
| Thrombocytosis |
|
|
|
|
|
(−) | 20.0 |
30.34±28.74 | 14.63 | 0.095 |
|
(+) |
| 13.51±3.97 | 11.55 |
|
| CEA (ref: 0–3.0
ng/ml) |
|
|
|
|
|
Normal | 24.0 |
26.79±25.97 | 13.88 | 0.274 |
|
High |
|
22.09±25.45 | 10.90 |
|
| CA 125 (ref: 0–11.1
U/ml) |
|
|
|
|
|
Normal |
7.7 |
26.66±25.64 | 13.83 | 0.501 |
|
High |
| 13.96±5.45 | 13.96 |
|
| CA 15–3 (ref:
0–31.3 U/ml) |
|
|
|
|
|
Normal | 19.4 |
22.17±18.14 | 13.88 | 0.031 |
|
High |
|
56.97±42.93 | 56.04 |
|
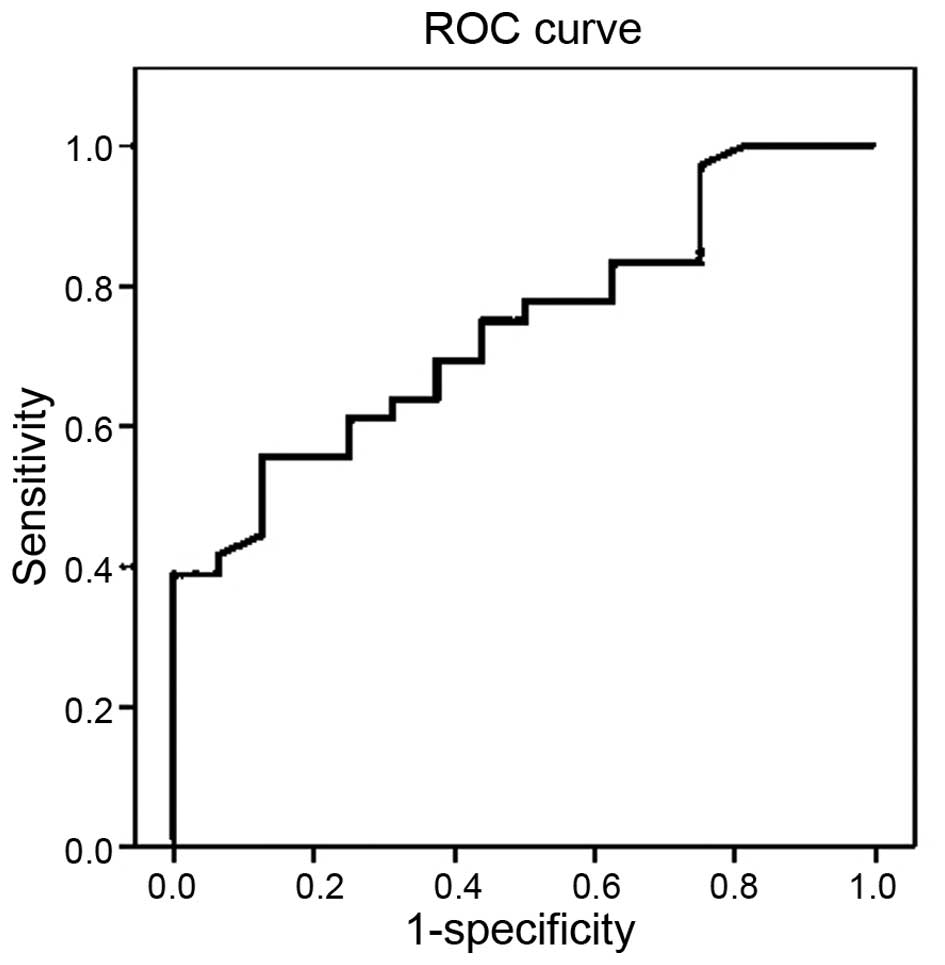
The cutoff value of HE4 levels for predicting breast
cancer was determined by ROC analysis. The cutoff value for the
prediction of breast cancer was determined at >13.24 pmol/l for
HE4 with a sensitivity of 61.11%, specificity of 68.75%, positive
predictive value of 81.48%, negative predictive value of 44.0% and
accuracy of 63.46% [AUC, 0.740 (95% CI, 0.604–0.875), P=0.006]
(Fig. 1).
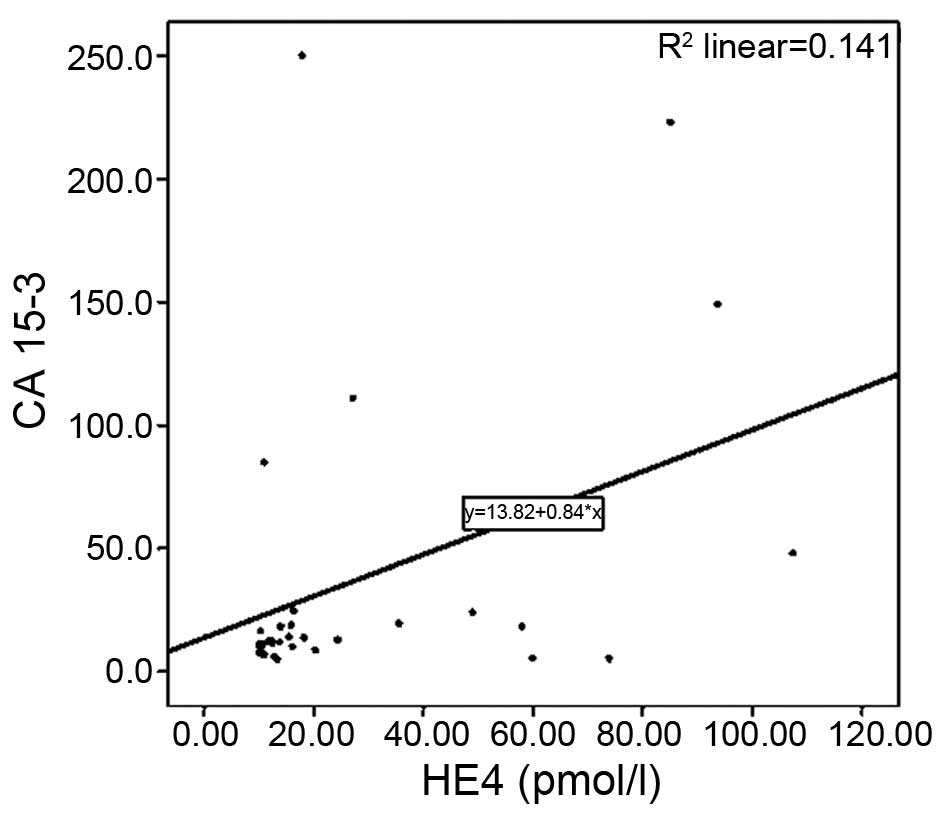
Furthermore, a positive correlation was determined
between the serum levels of HE4 and CA 15–3 in breast cancer
patients (r=0.399; P=0.026) (Fig.
2).
Discussion
To the best of our knowledge, the present study was
the first to determine the diagnostic value of serum HE4 for breast
cancer patients. Breast cancer is a heterogeneous group of diseases
that differs in their pathological characteristics and clinical
presentation. The risk of recurrence and prognosis are affected by
the stage at diagnosis and biological features of the tumor. The
main challenge regarding biomarkers for breast cancer diagnosis is
to improve the accuracy for the detection of the malignancy at the
earliest possible stage. Numerous serum markers, including BR 27.29
(CA 27.29), mucin-like carcinoma-associated antigen, CA 549 and
CEA, have been investigated; however, none of these markers have
reached the sensitivity and specificity required for standard
clinical practice (4).
According to American Society of Clinical Oncology
guidelines, CA 15–3 concentrations are increased in 10% of patients
with stage I of the disease, 20% in stage II, 40% in stage III and
75% in stage IV. Furthermore, CA 15–3 concentrations 5- to 10-fold
above the upper limit of the reference interval may alert a
physician to the presence of metastatic disease. However, a low
concentration does not exclude metastasis (12). In addition, Molina et al
(13) showed that the sensitivity of
CA 15–3 was 16–18% in patients with locoregional disease and 61–70%
in those with advanced disease. Due to its lack of specificity and
sensitivity with regards to breast cancer, CA 15–3 is not
recommended for either screening or early diagnosis.
HE4, also known as whey acidic four-disulfide core
domain protein 2 (WFDC2), is a protein encoded by the WFDC2 gene.
Due to similarities of HE4 with other whey acidic protein family
members, it has been implied that the protein may function as an
anti-proteinase (6,7). Furthermore, a recent study by LeBleu
et al (14) reported that HE4
functions as a serine protease inhibitor, decreasing the activity
of serine proteases Prss35 and Prss23, which degrade type I
collagen that accumulates in kidney fibrosis.
The potential use of HE4 as a tumor marker has been
supported by an increasing number of studies demonstrating an
upregulation of HE4 in a range of malignant neoplasms, particularly
of gynecological, pulmonary and gastrointestinal origin (6–9). The
serological detection of HE4 has been shown to have increased
sensitivity and specificity in the detection of ovarian cancer
compared with CA 125, which is the current gold standard serum
biomarker for ovarian carcinoma (7,15,16). As aforementioned, previous studies
have demonstrated the diagnostic and prognostic potential of the
serum levels of HE4 in several other cancer types, including those
of gynecological and gastrointestinal origin (7,9,15,16). While
all these studies indicated that the cutoff point for HE4 is 70–150
pmol/l for ovarian cancer, the prediction of HE4 for breast cancer
was determined as >13.24 pmol/l in the present study.
Galgano et al (6) reported the mRNA and protein expression
of HE4 in normal and malignant tissues. Positive HE4
immunoreactivity was present in ovarian cancer, as well as other
types of cancer, including lung, endometrial, breast and
gastrointestinal cancer and mesothelioma (6). The highest expression levels were found
in ovarian serous cancer, and significant positive staining was
identified in breast carcinoma tissues. However, malignant breast
tissue showed variable expression (6). In addition, Kamei et al (17) found that the increased expression of
HE4 in breast cancer tissues correlated with lymph node invasion
and was a possible predictive factor of breast cancer recurrence.
The five-year disease-free survival in the HE4-positive group
(58.6%) was significantly worse than that in the negative group
(85.6%). These findings indicated that HE4 is significant in
association with breast cancer. However, to the best of our
knowledge, the serum levels of HE4 in breast cancer patients, and
their diagnostic and prognostic potential have not been
investigated. In the present study, the serum levels of HE4 in
patients diagnosed with breast and ovarian cancer were assessed
prior to chemotherapy and compared with those in healthy
individuals. The serum levels of HE4 were significantly increased
in patients with breast and ovarian cancer compared with those in
healthy controls. However, multivariate analysis did not show any
significant positive correlation of HE4 serum levels with
histological grade, lymph node involvement and clinical stage in
breast cancer patients. Of note, the serum levels of HE4 were
positively correlated with the serum levels of CA 15–3 in patients
with breast cancer. The sensitivity of serum HE4 was 61.11% and the
specificity was 68.75% for distinguishing breast cancer patients
from healthy controls. These findings indicate that HE4 may be used
as a predictive marker for breast carcinoma.
The present study has certain limitations, the most
evident of which was the small sample size. In future studies, it
may be appropriate to compare the serum levels of HE4 with those in
tumor tissue since it would be of interest to ascertain the
distribution of the HE4 protein and/or mRNA in normal tissue,
ductal carcinoma in situ and invasive tumors, i.e. by
immunohistochemistry and polymerase chain reaction analysis. The
observations of the present study should be confirmed by future
studies using a larger cohort and additional tissue analyses.
In conclusion, the present study found a significant
elevation of serum HE4 levels in patients with breast cancer
compared with those in healthy controls. The preliminary results
indicate that HE4 may serve as a novel biomarker for breast cancer.
However, large-scale clinical studies are required to further
determine the predictive value of this biomarker.
References
|
1
|
Howlader N, Noone AM, Krapcho M, et al:
SEER Cancer Statistics Review, 1975–2011, National Cancer
Institute. Bethesda, MD: based on November 2015 SEER data
submission. http://seer.cancer.gov/archive/csr/1975_2011/Accessed.
December 17–2018
|
|
2
|
Geng B, Liang MM, Ye XB and Zhao WY:
Association of CA 15–3 and CEA with clinicopathological parameters
in patients with metastatic breast cancer. Mol Clin Oncol.
3:232–236. 2015.PubMed/NCBI
|
|
3
|
Ideo H, Hinoda Y, Sakai K, Hoshi I,
Yamamoto S, Oka M, Maeda K, Maeda N, Hazama S, Amano J and
Yamashita K: Expression of mucin 1 possessing a 3′-sulfated core1
in recurrent and metastatic breast cancer. Int J Cancer.
137:1652–1660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
5
|
Kirchhoff C, Habben I, Ivell R and Krull
N: A major human epididymis-specific cDNA encodes a protein with
sequence homology to extracellular proteinase inhibitors. Biol
Reprod. 45:350–357. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
7
|
Hellström I, Raycraft J, Hayden-Ledbetter
M, et al: The HE4 (WFDC2) protein is a biomarker for ovarian
carcinoma. Cancer Res. 63:3695–3700. 2003.PubMed/NCBI
|
|
8
|
Bingle L, Cross SS, High AS, et al: WFDC2
(HE4): A potential role in the innate immunity of the oral cavity
and respiratory tract and the development of adenocarcinomas of the
lung. Respir Res. 7:612006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neal RL, Nam KT, LaFleur BJ, et al:
Human epididymis protein 4 is up-regulated in gastric and
pancreatic adenocarcinomas. Hum Pathol. 44:734–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
The TNM Classification of Malignant
Tumours of the Union for International Cancer Control. Sobin LH,
Gospodarowicz MK and Wittekin C: (7th). Wiley-Blackwell. Hoboken:
2009.
|
|
11
|
World Health Organization (WHO).
Classification of Tumours of the Breast. Lakhani SR, Ellis IO,
Schnitt SJ, Tan PH and van de Vijver MJ: (4th). WHO Press.
2012.
|
|
12
|
Clinical practice guidelines for the use
of tumor markers in breast and colorectal cancer. Adopted on May
17, 1996 by the American Society of Clinical Oncology. J Clin
Oncol. 14:2843–2877. 1996.PubMed/NCBI
|
|
13
|
Molina R, Jo J, Filella X, Zanon G, Pahisa
J, Munoz M, Farrus B, Latre ML, Escriche C, Estape J, et al:
c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast
cancer: Prognostic value. Breast Cancer Res Treat. 51:109–119.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeBleu VS, Teng Y, O'Connell JT, Charytan
D, Müller GA, Müller CA, Sugimoto H and Kalluri R: Identification
of human epididymis protein-4 as a fibroblast-derived mediator of
fibrosis. Nat Med. 19:227–231. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferraro S, Braga F, Lanzoni M, Boracchi P,
Biganzoli EM and Panteghini M: Serum human epididymis protein 4 vs
carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic
review. J Clin Pathol. 66:273–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhen S, Bian LH, Chang LL and Gao X:
Comparison of serum human epididymis protein 4 and carbohydrate
antigen 125 as markers in ovarian cancer: A meta-analysis. Mol Clin
Oncol. 2:559–566. 2014.PubMed/NCBI
|
|
17
|
Kamei M, Yamashita S, Tokuishi K, Hashioto
T, Moroga T, Suehiro S, Ono K, Miyawaki M, Takeno S, Yamamoto S and
Kawahara K: HE4 expression can be associated with lymph node
metastases and disease-free survival in breast cancer. Anticancer
Res. 30:4779–4783. 2010.PubMed/NCBI
|