Cervical cancer in Peru
Cervical cancer is a public health problem
predominantly in developing countries where the incidence and
mortality rates are high. GLOBOCAN data regarding this malignancy
for Peru describe a total of 4,636 novel cases with 1,715
mortalities in 2012 (1). Peru is
located on the west coast of South America with ~31 million
inhabitants, where one-third of the total population lives in Lima,
the capital city. This concentration of population in only one city
contributed to the establishment of structural, infrastructural and
procedural barriers for the Peruvian health system.
The cervical cancer incidence varies across the
regions in Peru. Geographically, Peru have three well defined
regions by the Andean mountains, the coast, and the mountains and
the jungle, where the population from each region is exposed to
different environmental conditions and risk factors.
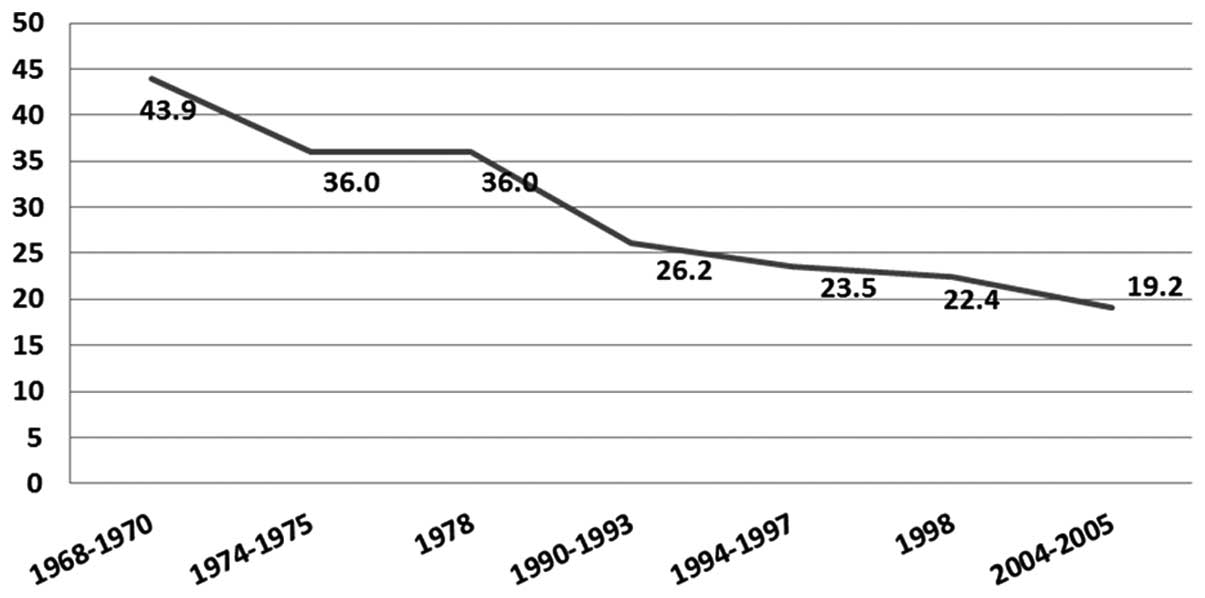
With regards to Lima, located in the coast of Peru,
data from the Metropolitan Cancer Registry describes a cervical
cancer age-standardized rate (ASR) incidence of 19.2 new cases per
100,000 women annually, quite different to the national incidence
that is 32.7 new cases per 100,000 women (2). This difference is explained by
lifestyles and socio-cultural inequalities between Lima and the
other regions of Peru. Notably, decades ago, Lima had a higher
incidence of cervical cancer where a progressive decrease through
the time was observed, while the incidence of breast cancer
continues to increase due to the process of epidemiological
transition (Fig. 1). On the other
hand, the Cancer Registry of Meteropolitan Lima describes different
cervical cancer incidences in the different districts of
Metropolitan Lima, where the higher income districts have the
lowest cervical cancer incidence (2).
With regards to other Peruvian cities, the latest
report of the Cancer Registry of Trujillo (located at north)
described an incidence ASR of 43.2 new cases per 100,000 women
annually in the period 1996–2002, showing a decrease compared with
the period 1991–1995 (incidence, 52.4). By contrast, the Cancer
Registry of Arequipa (South of Peru) reported an incidence of 43.2
new cases per 100,000 women annually in the period 2004–2007,
higher compared with that reported in the period 2002–2003 (35.2)
(3,4).
Unfortunately, no other cancer registries exist in Peru. The
differences in the decrease of cervical cancer incidence in other
Peruvian regions compared to Lima highlights disparities in
education and access to specialized healthcare services.
Conventional cytology (Pap) and visual
inspection following the application of acetic acid or white
vinegar (VIA) are currently used in primary screening, while HPV
molecular sub-typing is offered only by a few laboratories.
Epidemiology of HPV in Peru
Although the pathogenic role of HPV16, 18 and 32 is
well understood, other subtypes also have a pathogenic role in
cervical cancer. A report by Halec et al (5) describes the HPV subtypes 26, 53, 66, 67,
68, 70, 73 and 82 as the only infectious agents in several cervical
cancer samples, indicating their potential carcinogenic role. This
fact could estimate the prevalence of carcinogenic HPV. In an
analysis of 10,575 cases of invasive cervical cancer, where our
country included 770 cases, a frequency of 85% of samples positive
for HPV DNA were reported, and the most common HPV types were 16,
18, 31, 33, 35, 45, 52 and 58 (91% of all HPV DNA-positive cases)
(6). A time trend study revealed that
HPV16 and 18 were the most common in cervical cancer, with not
significant changes since 1940 (7).
With regards to HPV prevalence in Peru, earlier reports by Santos
et al (8) described that in a
population of Metropolitan Lima a prevalence of 95.3% was observed
in women with squamous cell carcinoma and in 92.0% in women with
adenocarcinoma/adenosquamous tumors (HPV 16, 18, 31, 52 and 35 were
most frequent), while the frequency the HPV infection in healthy
women was 17.7 (8). The TATI project
(Spanish acronym for ‘screening and immediate treatment’ of cervix
uteri lesions) that screened 5,435 women from the Peruvian jungle
(San Martin) in 2001 demonstrated a high-risk HPV frequency of
12.6% (9), while a recent report
evaluating 2,247 samples from screened women aged 17–79 years from
urban areas of Iquitos, Cajamarca, Piura, Chiclayo, Lima, Arequipa,
Cuzco and Juliaca described a high-risk HPV distribution of 34.5%.
Of these, 82.7% of cases had single infections and 17.3% had
multiple infections (10). A study by
Bautista et al (11) in 1,142
mothers from the social program ‘el vaso de leche’ of the Surquillo
District in the region of Lima found a prevalence of high-risk HPV
of 15.2% (11). With a small sample
size of female sex workers (n=87), Montano et al (12) reported a 50.6% of infection with HPV
with a 35.6% of infection with high-risk HPV, while Brown et
al (13) found in 184 female sex
workers (18–26 years old) a 65.8% rate of HPV infection in cervical
samples, where 8.3% of these women also exhibited oral HPV
(12,13).
Initiatives for cancer control in Peru
The cancer control first initiative in Peru was
launched 1935 with taxes on ice and refrigerator devices with the
aim of collecting money to build the first cancer hospital. This
was inaugurated in 1939 offering treatment, predominantly
radiotherapy. In 1985, the first policies for cancer control were
developed in the Peruvian National Cancer Institute. The first
initiative for a massive plan for prevention of gynecological
cancer was launched in 1998 for breast and cervical cancer, while
in 2000 the first guidelines for cervical cancer prevention,
including the use of pap test as screening method and
cryotherapy for treatment, were published. In 2002, the Program for
Health Promotion and Cancer Control was launched. With regards to
the concern about the public health problem that cancer represents,
in 2005 the Multisectoral Coalition ‘Peru against Cancer’, a
multi-institutional initiative of Peruvian Institutions with the
support of the American Cancer Society, the Union for International
Cancer Control, the World Health Organization/Pan American Health
Organization and the Washington Cancer Institute was created. This
led to the development of the First National Plan for Cancer
Control launched in 2006 under the title ‘Strategic Plan
2006–2016′. In 2007, a landmark in Latin America was established
with the launch of the first Comprehensive National Cancer Control
Plan, where treatment of low-income patients was for free (covered
by a Comprehensive Health Insurance). This Cancer Control Plan was
performed under a strategic plan of ‘budget by results’, which
means more objectives accomplished and more money for funding. In
2012, the current cancer control plan named ‘Plan Esperanza’ (‘Hope
Plan’ in Spanish) was launched, which also promotes and supports
cancer advisory groups; the social promotion of cancer prevention
and development of training programs for thousands of health
professionals across the country (14). In 2014, the ‘Plan Esperanza’ provided
cervical cancer screening to >2.3 million women in Peru
(15).
Barriers for cervical cancer control
In Peru, well-identified geographical, cultural,
structural, infrastructural and procedural barriers exist for plans
of cervical control cancer. Disparities also exist between the
regions, predominantly due to the centralization and concentration
of population in the coast. With regards to cultural barriers,
fears and concerns remain a problem for the gynecological
examination, including the sampling for the pap smear.
Medrano et al (16)
demonstrated, in a survey of 225 women attending the public clinic
in Los Olivos district, that the fear and shame of the examination,
neglect to attend to the gynecological control and laziness to take
the screening were factors identified for the non-compliance of the
cervical cancer screening (16).
Support from husbands or partners is also important for individuals
to attend their screen (17). There
are few trained specialists in cancer screening and this is more
evident in remote villages, and educational levels are higher in
the coast compared with the mountains and jungle (18,19).
A previous study by Paz-Soldan et al
(20) in 6,712 women between 18 and
29-years-old in 20 Peruvian cities demonstrated by multivariate
analysis that women who are more likely to have a pap smear were
those with high levels of education, were married/co-habiting, were
at lower age at first intercourse and living by the coast (20).
Since 2007 when the process of decentralization of
cancer care was initiated, the current infrastructure is not enough
to cover the nationwide demand. A wide gap exists between supply
and demand of laboratories, cytopathologists and centers for the
management of dysplasias and cancer detection. By contrast, the
social structure of remote villages may provide a real barrier to
health care intervention at these locations, where leaders of the
communities must be identified and involved.
Approaches to improve the early detection of
cervical cancer in Peru
The self-sampling test for molecular prevention of
HPV has novel devices with similar sensitivity to a direct
endocervical specimen obtained by a physician. This approach is
being used in the cervical cancer control strategies, predominantly
in locations where cultural factors are identifiable or where there
are long distances between the home of patients and the laboratory
(or hospital) facilities (21). In a
community-based participatory research (CBPR) in the Peruvian
Jungle, Abuelo et al (22),
described 99.7% of high satisfaction with the self-sampling for HPV
detection, while, 100% of women invited to be involved (n=320) gave
an HPV sample (22). In other
community-based participatory research peformed in Manchay, a city
near to Lima, it was demonstrated that the health promoters from
the community had an important role in the success of the project,
where 97% of registered participants gave an HPV sample and 90% of
women returned for a 6-month follow-up (23).
The insurance coverage appears to be another factor
affecting the realization of the pap test. A cross-sectional
test by Barrionuevo-Rosas et al (24), in a sample of 12,272 women aged
30–49-years-old demonstrated that women without insurance were the
least likely to be screened compared with patients with public
insurance (odds ratio=1.27; 95% confidence intervals, 1.24–1.31)
and patients with private insurance, (odds ratio=1.52; 95%
confidence intervals, 1.46–1.58) (24).
With regards to high-grade HPV detection, several
molecular methods must be evaluated in a cost-effective manner.
There is an experience in Peru with self-sampling and solid media
specimen cards and with centralized high-throughput processing with
MALDI-TOF, where the samples were sent to China for analysis and
the results were delivered 4 days later (25). This technology must be implemented in
strategic regions and can also be cost-effective if laboratories
process a great number of samples.
Is massive HPV vaccination suitable for the
Peruvian population?
Vaccination campaigns must also be established with
community involvement in order to gain high rates of adherence to
cycle dose schedules. In a CBPR, 318 girls from Iquitos (Peruvian
jungle) assented for vaccination, 98% received the first dose of
vaccine, 88 and 65% received the second and third doses,
respectively. Completion rates in this study were two times higher
than reported in other countries (26). Another interesting strategy is
implementation of school-based vaccination programs, which was
shown feasible in Peru (27) and
where the experience in Brazil demonstrated a three-dose completion
rate of 97.2%, with a parental acceptance rate of 91.8% (28). In addition to these strategies, it is
important to develop nationwide programs of formative research to
identify and overcome local barriers to developing and performing
successfully vaccination programs (29). By contrast, Gutierrez-Aguado (30) demonstrated that in Peru, HPV
vaccination can be cost-utility compared with not vaccinating, with
an incremental cost-utility of 6775 USD/QALY (30). In 2015, the Ministry of Health planned
to vaccinate 475,000 girls (31).
Challenges for the future
In Peru, it is possible to decrease the incidence of
cervical cancer by detecting high-grade HPV and/or intraepithelial
cervical neoplasms. Several efforts from the government,
Non-governmental organizations and individual researchers have
determined the epidemiology and identified several barriers and
future challenges to reduce the cervical cancer burden in Peru
(Table I).
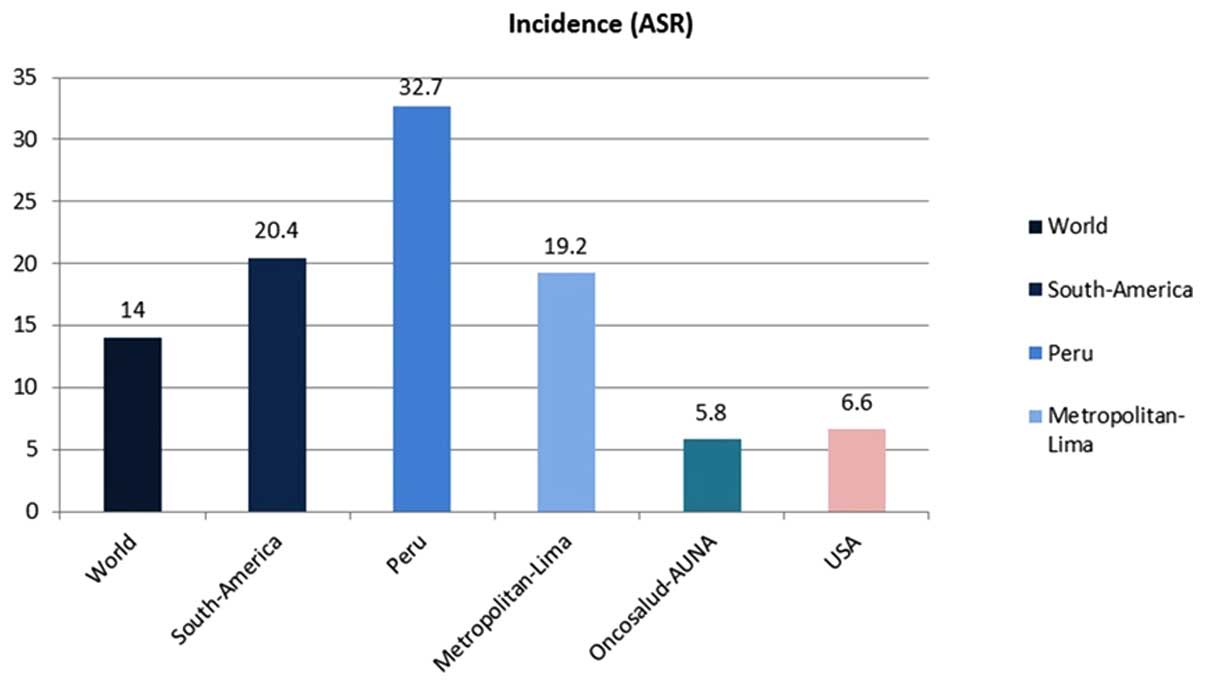
The case of the Cohort of Oncosalud is interesting.
With ~750,000 affiliates enrolled in a pre-paid system for cancer
care (around half are women), the incidence of cervical cancer is
5.8/100,000 women, a lower rate compared with the national
incidence (32.7/100,000) or the incidence to Metropolitan Lima
(19.2/100,000; Fig. 2). The
affiliates to Oncosalud are predominantly in the A and B
socioeconomic status, and in the context of cancer prevention in
this group, screening for free is offered, including mammograms,
pap smear and colposcopy for women. By contrast to the low
incidence of cervical cancer, a higher incidence compared with the
national values is observed in breast cancer (52.7/100,000 vs.
28/100,000 women).
The funding for cancer care is important to improve
the outcomes of cervical cancer. In 2014, 64.31% of patients who
attended the National Cancer Institute of Peru were covered by
Comprehensive Health Insurance in the context of ‘Plan Esperanza’
(15). However, the level of coverage
for cancer in Lima should improve, since 41% of the national budget
for cervical cancer screening is being spent in Lima, according to
the Ministry of Economy and Finances data (32).
Establishment of Cancer Preventoria has provided the
infrastructure to empower the screening strategies for the early
detection of malignancies, predominantly for breast and cervical
cancer. These services should be massively widespread and in
addition, the involvement of the mass media in highly important in
order to educate the population.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, et al: GLOBOCAN 2012 v1.0. cancer
incidence and mortality worldwide: IARC Cancer Base No. 11
[Internet]. Lyon, France: International Agency for Research on
Cancer. 2013.http://globocan.iarc.frAccessed. July
01–2015
|
|
2
|
Cancer Registry of Metropolitan Lima.
2014.
|
|
3
|
Albújar PF: Cancer in Trujullo, 1996–2002:
Incidence and mortality. Population Cancer Registry of Areguipa
(Perú). 2006.
|
|
4
|
Ministerio de Salud: Registro de cáncer
poblacional de arequipa. Perú. Arequipa: Instituto Regional de
Enfermedades Neoplásicas del Sur. 2011.
|
|
5
|
Halec G, Alemany L, Lloveras B, Schmitt M,
Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimerà N, Grabe N, et
al: Pathogenic role of the eight probably/possibly carcinogenic HPV
types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J
Pathol. 234:441–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alemany L, de Sanjosé S, Tous S, Quint W,
Vallejos C, Shin HR, Bravo LE, Alonso P, Lima MA, Guimerà N, et al:
Time trends of human papillomavirus types in invasive cervical
cancer, from 1940 to 2007. Int J Cancer. 135:88–95. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santos C, Muñoz N, Klug S, Almonte M,
Guerrero I, Alvarez M, Velarde C, Galdos O, Castillo M, Walboomers
J, et al: HPV types and cofactors causing cervical cancer in Peru.
Br J Cancer. 85:966–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almonte M, Ferreccio C, Gonzales M,
Delgado JM, Buckley CH, Luciani S, Robles SC, Winkler JL, Tsu VD,
Jeronimo J, et al: Risk factors for high-risk human papillomavirus
infection and cofactors for high-grade cervical disease in Peru.
Int J Gynecol Cancer. 21:1654–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwasaki R, Galvez-Philpott F, Arias-Stella
J Jr and Arias-Stella J: Prevalence of high-risk human
papillomavirus by cobas 4800 HPV test in urban Peru. Braz J Infect
Dis. 18:469–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bautista F, Vallejos C, Bances G, Galdos O
and Santos C: Prevalence ofcervical and HPV infection in women of
the “Milk Glass” comitee in Surquillo District. Carcinos. 1:3–9.
2013.
|
|
12
|
Montano SM, Hsieh EJ, Calderón M, Ton TG,
Quijano E, Solari V and Zunt JR: Human papillomavirus infection in
female sex workers in Lima, Peru. Sex Transm Infect. 87:81–82.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown B, Blas MM, Cabral A, Carcamo C,
Gravitt PE and Halsey N: Oral sex practices, oral human
papillomavirus and correlations between oral and cervical human
papillomavirus prevalence among female sex workers in Lima, Peru.
Int J STD AIDS. 22:655–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vallejos C: National plan for prevention,
early detection, and cancer control in Peru. Am Soc Clin Oncol Educ
Book. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Cancer Institute of Peru. Main
results of ‘Hope Plan’. 2014.http://www.inen.sld.pe/portal/documentos/pdf/institucional/Memoria/19052015_PRINCIPALES%20RESUL%20%20DEL%20INEN%20%20PARA%20PLAN%20ESPERANZA%20Diciembre%202014%2020012015.pdfAccessed.
August 11–2015
|
|
16
|
Medrano MM: Sociocultural and
psychological factors that influence the decision of users for
taking Pap smear. Carlos Cueto Fernandini health center. Year 2014.
Thesis. Faculty of Obstetrics. Universidad Nacional Mayor de San
Marcos.
|
|
17
|
Winkler JL, Lewis K, Del Aguila R,
Gonzales M, Delgado JM, Tsu VD and Sellors JW: Is magnification
necessary to confirm visual inspection of cervical abnormalities? A
randomized trial in Peru. Rev Panam Salud Publica. 23:1–6. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zevallos L, Pastor R and Moscoso B: Supply
and demand of medical specialists in the health facilities of the
ministry of health: National, regional and by type of specialty
gaps. Rev Peru Med Exp Salud Publica. 28:177–185. 2011.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elias M and Rey S: Educational performance
and spatial convergence in Peru. Région et Développement.
33:107–135. 2011.
|
|
20
|
Paz Soldan VA, Lee FH, Carcamo C, Holmes
KK, Garnett GP and Garcia P: Who is getting Pap smears in urban
Peru? Int J Epidemiol. 37:862–869. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Belinson JL, Du H, Yang B, Wu R, Belinson
SE, Qu X, Pretorius RG, Yi X and Castle PE: Improved sensitivity of
vaginal self-collection and high-risk human papillomavirus testing.
Int J Cancer. 130:1855–1860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abuelo CE, Levinson KL, Salmeron J,
Sologuren CV, Fernandez MJ and Belinson JL: The Peru cervical
cancer screening study (PERCAPS): The design and implementation of
a mother/daughter screen, treat, and vaccinate program in the
Peruvian jungle. J Community Health. 39:409–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levinson KL, Abuelo C, Chyung E, Salmeron
J, Belinson SE, Sologuren CV, Ortiz CS, Vallejos MJ and Belinson
JL: The Peru cervical cancer prevention study (PERCAPS):
Community-based participatory research in Manchay, Peru. Int J
Gynecol Cancer. 23:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrionuevo-Rosas L, Palència L and
Borrell C: How does type of health insurance affect receipt of Pap
testing in Peru? Rev Panam Salud Publica. 34:393–400. 2013.(In
Spanish). PubMed/NCBI
|
|
25
|
Levinson KL, Abuelo C, Salmeron J, Chyung
E, Zou J, Belinson SE, Wang G, Ortiz CS, Vallejos CS and Belinson
JL: The Peru cervical cancer prevention study (PERCAPS): The
technology to make screening accessible. Gynecol Oncol.
129:318–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Widdice LE, Bernstein DI, Leonard AC,
Marsolo KA and Kahn JA: Adherence to the HPV vaccine dosing
intervals and factors associated with completion of 3 doses.
Pediatrics. 127:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Penny M, Bartolini R, Mosqueira NR,
LaMontagne DS, Mendoza MA, Ramos I, Winkler JL, Villafana J,
Janmohamed A and Jumaan AO: Strategies to vaccinate against cancer
of the cervix: Feasibility of a school-based HPV vaccination
program in Peru. Vaccine. 29:5022–5030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fregnani JH, Carvalho AL, Eluf-Neto J,
Ribeiro Kde C, Kuil Lde M, da Silva TA, Rodrigues SL, Mauad EC,
Longatto-Filho A and Villa LL: A school-based human papillomavirus
vaccination program in barretos, Brazil: Final results of a
demonstrative study. PLoS One. 8:e626472013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartolini RM, Winkler JL, Penny ME and
LaMontagne DS: Parental acceptance of HPV vaccine in Peru: A
decision framework. PLoS One. 7:e480172012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gutiérrez-Aguado A: Cost-utility of the
vaccine against the human papiloma virus in peruvian women. Rev
Peru Med Exp Salud Publica. 28:416–425. 2011.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ministry of Health of Peru. Press Note.
http://www.minsa.gob.pe/?op=51¬a=16252Accessed.
August 05–2015
|
|
32
|
Ministry of Economy and Finance of Peru.
Economic transparency. http://apps5.mineco.gob.pe/transparency/Browser/Navegar.aspxAccessed.
March 11–2016
|