Introduction
Adenoid cystic carcinoma (ACC) was first described
by Spies in 1930 (1). It constitutes
a rare, malignant tumor, reportedly representing <1% of all head
and neck cancers. ACC may arise at various sites in the body,
including the salivary glands. However, the minor salivary glands
of the oral cavity are the most common site, comprising 60% of all
cases (2).
ACC typically grows slowly, but carries a high
overall recurrence rate (≤53%) after initial primary treatment. The
risk of recurrence increases in cases with perineural or vascular
invasion, positive resection margins and solid histological
characteristics. Moreover, the recurrence rate is generally higher
in women (3). Evidence of vascular
invasion and positive margins may be used as predictors of tumor
recurrence (2,4,5).
As the presence of this tumor at sites other than
the head and neck is quite rare, and since the progression of the
disease is occasionally indolent, accurateand timely diagnosis may
be difficult.
In this report, we present a rare case of upper
tracheal/subglottic ACC mimicking thyroid carcinoma at presentation
and the detailed diagnostic process that led to treatment.
Case report
A 47-year-old woman with known hypothyroidism
presented with a history of gradual progressive neck swelling for 6
months, a cough persisting for 4 months, shortness of breath and
hoarseness for 1 week. The patient was referred to the Department
of Otolaryngology at King Fahad Medical City (Riyadh, Saudi
Arabia). As part of the diagnostic investigations, the patient
underwent flexible bronchoscopy that revealed no abnormal findings.
The pulmonologist at King Fahad Medical City diagnosed the patient
with asthma, and treated the respiratory symptoms with
bronchodilators and inhaled steroids. The patient experienced some
improvement, but her symptoms were not completely eliminated. The
patient had no history of weight loss, dysphagia, pain, or exposure
to radiation, and no family history of malignancy. In addition to
antiasthmatic inhalers, the patient was prescribed levothyroxine
(50 µg daily) and antihypertensive medications (amlodipine, 10
mg).
Physical examination revealed a firm midline mass
with smooth, regular borders, sized ~3×4 cm, with no palpable
cervical lymph nodes. The mass was located right below the level of
the cricoid cartilage and moved with swallowing. Flexible
laryngoscope examination revealed left vocal cord paralysis.
Routine laboratory investigations, including thyroid function
tests, were within the normal limits. An ultrasound revealed a
normal-sized right thyroid lobe, with a left lobe measuring
1.7×1.6×4.4 cm. Although both lobes exhibited heterogeneous
echotexture, it was more prominent in the left lobe, which
demonstrated increased vascularity with no discrete nodules.
Fine-needle aspiration (FNA) of the thyroid reported findings
consistent with follicular neoplasm/suspicious for follicular
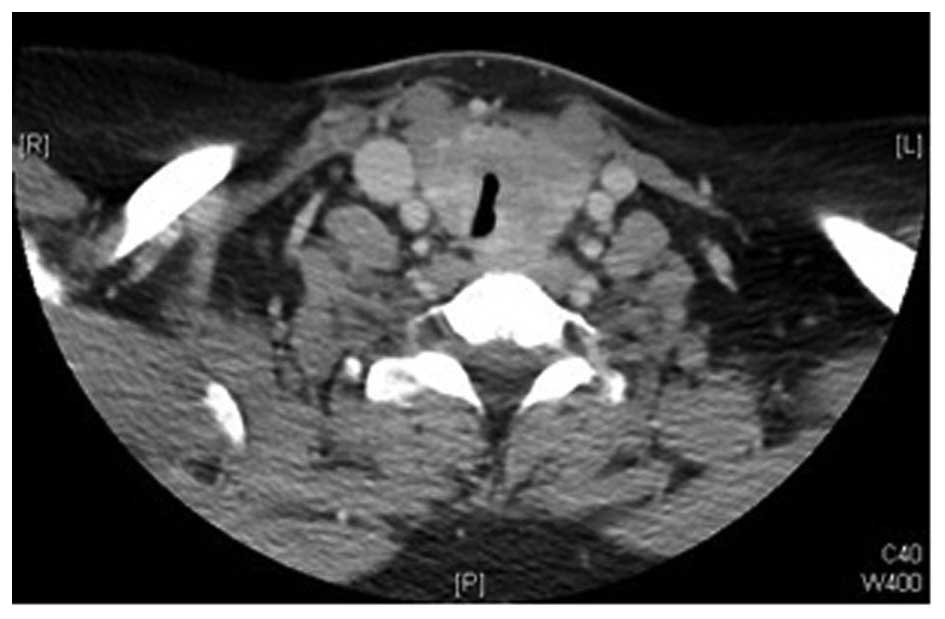
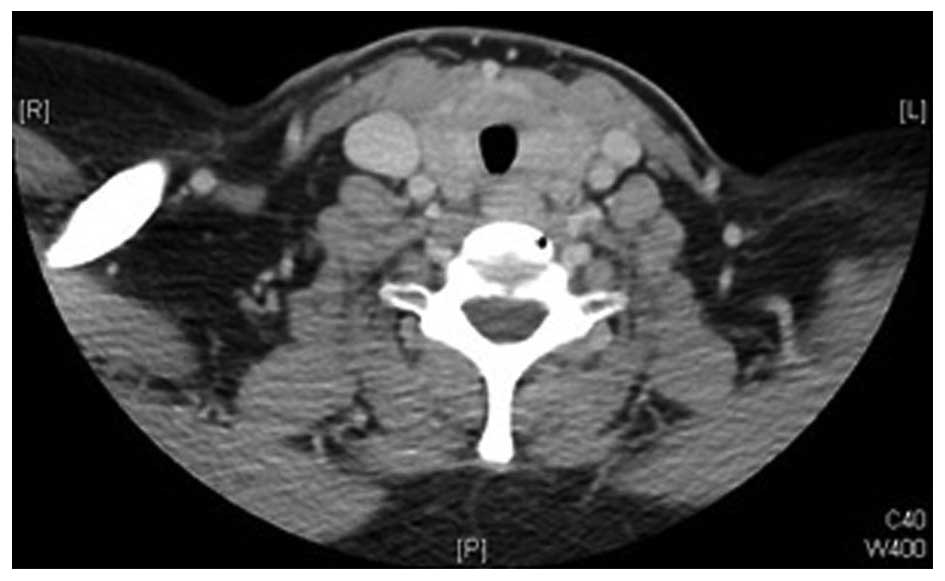
neoplasm. Computed tomography (CT) revealed diffuse enlargement of
the thyroid gland, more prominent on the left side, with
significant tracheal compression and possible underlying
infiltrative processes, but no cervical lymphadenopathy (Figs. 1 and 2).
Based on the FNA and imaging results, the patient was scheduled to
undergo total thyroidectomy and possible tracheal resection.
Intraoperatively, the thyroid lobes were small and hard, with no
gross invasion of the recurrent laryngeal nerve, apparent gross
invasion of the trachea, and surrounding edema. As the
intraoperative findings were atypical of thyroid carcinoma, frozen
specimen sections from the tracheal wall, thyroid gland and left
recurrent laryngeal nerve were analyzed. The results reported
poorly differentiated carcinoma with adenoid cystic features in the
tracheal wall and thyroid gland. The left recurrent laryngeal nerve
was reported negative for malignancy.
The surgeons decided to close and plan a
second-stage operation after receiving the final surgical pathology
report and discussing the results with the patient. The final
pathology report confirmed the diagnosis of ACC with predominant
tubular and cribriform patterns, with a positive tracheal resection
margin and evidence of perineural invasion. The abdominal and chest
CT scans, as well as a positron emission tomography scan, were
negative. The patient was subsequently rescheduled to undergo total
laryngectomy with bilateral neck dissection and tracheal resection.
Intraoperatively, the larynx, left side of the cricoid cartilage
and the first two tracheal rings were found to be infiltrated by
ACC. Total laryngectomy with partial pharyngectomy and tracheal
resection with bilateral neck dissection were completed without
complications. The final pathology confirmed the diagnosis of ACC
involving the larynx and trachea. The epicenter of the tumor was
located in the subglottic region and upper trachea, with extensive
submucosal tumor formation involving the entire left hemilarynx.
However, all regional lymph nodes were negative for malignancy. The
final diagnosis was locally advanced ACC of the trachea, stage
T4N0M0.
Discussion
Rare tumors are difficult to diagnose, particularly
when their location is uncommon. Tracheal tumors are classified
into three categories, namely benign, primary malignant and
secondary malignant. Primary malignant tumors most frequently
originate from the epithelium or glandular tissues of the trachea.
It is extremely rare for these tumors to arise from the connective
tissue of the trachea. The two most common types of epithelial
tumors are squamous cell carcinoma and adenoid cystic carcinoma,
which occur in generally equal proportions. Squamous cell carcinoma
is more common in men, while ACC does not show any gender
predilection. Pathologically, squamous cell carcinoma is believed
to arise from the tall columnar pseudostratified epithelium of the
trachea, whereas ACC arises from the mixed seromucinous glands
within the trachea (6).
Patients with ACC may present with a slowly
progressive shortness of breath and cough, often misdiagnosed as
asthma due to the slow rate of ACC progression. In advanced cases,
hemoptysis or stridor may be the main diagnostic characteristics.
Some patients may also present with hoarseness and weight loss
(7). Although rarely, patients may
also present with midline neck swelling (8,9). Due to
its non-specific symptoms and slow progression, the mean interval
between symptom presentation and correct diagnosis of ACC has been
reported to be 12 months (10). Azar
et al reported 6 cases of tracheal ACC; dyspnea and
non-productive cough were the most frequent complaints, and the
duration of symptoms prior to final diagnosis ranged from 6 to 24
months. Although our patient presented with classic, non-specific
clinical findings, a follicular pattern in the FNA led to the
initial diagnosis of malignant thyroid neoplasm (11).
The clinical and pathological characteristics of
tracheal ACC were first reported by Billroth in 1859 (7,12). Despite
their rarity, these tumors are the most common type of salivary
gland neoplasms in the lungs (13).
ACC commonly occurs in the trachea or main stem bronchi. ACC
characteristics may be found anywhere along the tracheal structure,
although they tend to be concentrated in the upper region.
Histologically, salivary gland ACC is identical to ACC originating
elsewhere, with both often demonstrating a tendency towards
submucosal extension.
On microscopic examination, cytological smears
consisted of small cells with hyperchromatic nuclei and
eosinophilic cytoplasm. However, relying on these characteristics
as the only diagnostic criterion may lead to a misdiagnosis,
particularly when ACC is encountered outside the salivary glands,
as was the case with our patient (9).
The FNA in our patient revealed alterations in the follicular cell
architecture characterized by cell crowding, microfollicles and
dispersed isolated cells, with some atypical cells containing a
scant amount of colloid. These characteristics supported the
diagnosis of follicular thyroid neoplasm. However, this
misdiagnosis may be attributed to the fact that the FNA specimen
was collected from the part of the tumor invading the thyroid
gland. Immunohistochemical staining for thyroglobulin or calcitonin
may help confirm the diagnosis of thyroid neoplasms; however, these
procedures are not routinely performed without clinical suspicion
of other possible diagnoses. There are three recognized
histological subtypes, namely tubular, cribriform and solid, with
the cribriform type being the most common.
One classic feature of ACC is frequent perineural
invasion (14), accounting for an
increased likelihood of positive microscopic surgical margins at
tracheal resection. Regional nodal metastasis has been reported to
be ~10%. Over 50% of patients with tracheal ACC develop
hematogenous spread, commonly to the lungs (11). In the present case, however, the
patient's chest, abdomen and pelvic preoperative CT scans revealed
no distant metastases.
Surgical resection is the treatment of choice for
tracheal tumors, whether benign or malignant. The type of surgery
is determined based on the location and extent of the tumor. The
standard surgery for ACC is sleeve resection of the trachea with
primary tracheal anastomosis (15).
When ACC is located in the upper trachea, tracheal resection
combined with total laryngectomy or laryngopharyngectomy may be
necessary, after which a permanent tracheal stoma is required. When
ACC involves the carina and main bronchi, a variety of carinal
resections and reconstructions are required, involving more
intricate surgical techniques. Cricotracheal resection may also be
performed in the presence of a subglottic tumor extending to the
proximal trachea. Surgical complications usually relate to the
extent of the surgery and the clinical status of the patient, and
may include anastomotic or wound dehiscence, tracheoesophageal
fistula, pharyngeal or esophageal leaks, vocal cord palsy, or
dysphagia due to pharyngeal stricture. However, such complications
and the resultant mortality have decreased significantly in last
decade (16).
Radiotherapy is usually recommended for incomplete
resections or positive margins, as it may provide additional local
disease control (7,14). The recommended dose is 54–60 Gy
(17). Silverman et al
(18) performed a retrospective study
to determine the benefits of postoperative radiation therapy in
patients with ACC of the head and neck. Their results indicated
that the addition of postoperative radiation therapy may improve
overall survival in patients with advanced T-stage tumors and
improve locoregional control in patients with microscopically
positive margins. However, the therapy showed no demonstrable
benefit in patients with negative margins.
As our patient was diagnosed with an advanced
T-stage tumor (T4), she received a radical dose of radiotherapy of
64 Gy using the intensity-modulated radiation therapy technique
(18).
Combined-modality therapy with surgery and
postoperative radiation may improve locoregional control,
particularly in advanced-stage disease. Published reports on
locoregional control rates for ACC treated with surgery alone range
from 40 to 46% at 5 years and from 21 to 25% at 10 years, compared
with the locoregional control rates for ACC treated with surgery
and postoperative radiation (64–95% and 68–83%, respectively).
Patients with stage III or IV tumors are most likely to benefit
from combined-modality therapy, with locoregional control rates of
16.8% with surgery alone vs. 51.3% with surgery and radiation
(18). The majority of clinicians
hypothesize that the additional benefit of postoperative radiation
therapy of ACC of the head and neck would translate into the same
benefit when used in the treatment of tracheal ACC. However, no
randomized control studies have shown any additional benefit with
adjuvant radiotherapy.
The overall survival rate of tracheal ACC has been
reported to range between 65–79% at 5 years and 53–57% at 10 years
(19). Gessrted et al reported
a 5-year survival rate of 52.4% for resected ACC and 33.3% for
unresectable ACC (16). Oplatek et
al reported that patients with advanced-stage tumors had a
lower median 10-year survival rate compared with patients with
stage I or II tumors. The median survival rate was 68 and 61 months
for stage III and IV disease, respectively (20).
It remains unclear whether treatment of tracheal ACC
with chemotherapy, either as adjuvant or mainstay treatment,
carries any clear benefit (7).
Our patient had a T4-stage tumor with perineural
invasion and a high risk of recurrence requiring close follow-up.
Accordingly, she was followed up for >1 year, without any
evidence of recurrence.
In conclusion, the case presented herein should
alert clinicians to the rare presentation of thyroid masses that do
not primarily originate from the thyroid gland. Thorough evaluation
of such masses may result in early diagnosis and timely, more
effective treatment.
Acknowledgements
The present study was supported by the College of
Medicine Research Center, Deanship of Scientific Research, King
Saud University, Kingdom of Saudi Arabia.
References
|
1
|
Spies JW: Adenoid cystic carcinoma:
Generalized metastases in three cases of basal cell type. Arch
Surg. 21:365–404. 1930. View Article : Google Scholar
|
|
2
|
Zhang CY, Xia RH, Han J, Wang BS, Tian WD,
Zhong LP, Tian Z, Wang LZ, Hu YH and Li J: Adenoid cystic carcinoma
of the head and neck: Clinicopathologic analysis of 218 cases in a
Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol.
115:368–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dantas AN, Morais EF, Macedo RA, Tinôco JM
and Morais Mde L: Clinicopathological characteristics and
perineural invasion in adenoid cystic carcinoma: A systematic
review. Braz J Otorhinolaryngol. 81:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honings J, Gaissert HA, Weinberg AC, Mark
EJ, Wright CD, Wain JC and Mathisen DJ: Prognostic value of
pathologic characteristics and resection margins in tracheal
adenoid cystic carcinoma. Eur J CardiothoracSurg. 37:1438–1444.
2010. View Article : Google Scholar
|
|
5
|
Haddad A, Enepekides DJ, Manolidis S and
Black M: Adenoid cystic carcinoma of the head and neck: A
clinicopathologic study of 37 cases. J Otolaryngol. 24:201–205.
1995.PubMed/NCBI
|
|
6
|
Macchiarini P: Primary tracheal tumours.
Lancet Oncol. 7:83–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang PY, Liu MS, Chen CH, Lin CM and Tsao
TC: Adenoid cystic carcinoma of the trachea: A report of seven
cases and literature review. Chang Gung Med J. 28:357–363.
2005.PubMed/NCBI
|
|
8
|
Kattepur AK, Patil D, Srinivas KG, Swamy
S, Murthy V, Amarendra S and Gopinath KS: Adenoid cystic carcinoma
of trachea masquerading as papillary thyroid cancer - a case
report. Case Rep Clin Pathol. 1:p532014.
|
|
9
|
Idowu MO, Reiter ER and Powers CN: Adenoid
cystic carcinoma: A pitfall in aspiration cytology of the thyroid.
Am J Clin Pathol. 121:551–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagheri R, Fatahi MS, Mokhtari AM and
Rahim MB: Adenoid cystic carcinoma of the trachea. Tanaffos.
7:49–54. 2008.
|
|
11
|
Azar T, Abdul Karim FW and Tucker HM:
Adenoid cystic carcinoma of the trachea. Laryngoscope.
108:1297–1300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan AJ, DiGiovanna MP, Ross DA, Sasaki
CT, Carter D, Son YH and Haffty BG: Adenoid cystic carcinoma: A
retrospective clinical review. Int J Cancer. 96:149–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vigg A, Mantri S and Vigg A and Vigg A:
Adenoid cystic carcinoma of trachea. Indian J Chest Dis Allied Sci.
46:287–290. 2004.PubMed/NCBI
|
|
14
|
Bonner Millar LP, Stripp D, Cooper JD,
Both S, James P and Rengan R: Definitive radiotherapy for
unresected adenoid cystic carcinoma of the trachea. Chest.
141:1323–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki T: What is the best management
strategy for adenoid cystic carcinoma of the trachea? Ann Thorac
Cardiovasc Surg. 17:535–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaissert HA, Grillo HC, Shadmehr MB,
Wright CD, Gokhale M, Wain JC and Mathisen DJ: Long-term survival
after resection of primary adenoid cystic and squamous cell
carcinoma of the trachea and carina. Ann Thorac Surg. 78:1889–1896;
discussion 1896–1897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lay G, Amichetti M, Dessi M, Orru S and
Versace R: Adenoid cystic carcinoma (ACC) of the tracheo-bronchial
tree treated with laser therapy and irradiation: Report of two
cases. The Open Lung Cancer Journal. 2:31–34. 2009. View Article : Google Scholar
|
|
18
|
Silverman DA, Carlson TP, Khuntia D,
Bergstrom RT, Saxton J and Esclamado RM: Role for postoperative
radiation therapy in adenoid cystic carcinoma of the head and neck.
Laryngoscope. 114:1194–1199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maziak DE, Todd TR, Keshavjee SH, Winton
TL, Van Nostrand P and Pearson FG: Adenoid cystic carcinoma of the
airway: Thirty-two-year experience. J Thorac Cardiovasc Surg.
112:1522–1532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oplatek A, Ozer E, Agrawal A, Bapna S and
Schuller DE: Patterns of recurrence and survival of head and neck
adenoid cystic carcinoma after definitive resection. Laryngoscope.
120:65–70. 2010.PubMed/NCBI
|