Introduction
Ovarian tumor is the most common female tumor type.
Epidemiological data indicates a steady rise of the incidence rate
in Europe and Asia. In the USA, the mortality of ovarian cancer is
~67.7% annually (1). The histological
type of most ovarian tumors is serous ovarian tumor. Ovarian serous
carcinomas account for 70% of all ovarian carcinomas. Therefore,
further research on ovarian tumors is required so that treatment
can be successful. Paired box gene (PAX)2 belongs to the PAX
family, consisting of nine members, PAX1-9. The genes of the PAX
family have a conserved DNA sequence motif, which comprises a 128
amino acid domain in the amino-terminal portion of the protein
(2). The role of PAX2 is crucial to
embryogenic development, morphogenesis and organogenesis (3). PAX2 overexpression is associated with
tumor types, including renal cell carcinoma, breast cancer and
ovarian cancer (4–6). Estrogens influence numerous
physiological processes in mammals, which mediates its effects
through the estrogen receptor (ER). The ER exists in two
predominant forms, ERα and ERβ. ERα is used as a therapeutic target
and the presence of ERα may be effective for endocrine therapy of
tumors. It has been previously reported that PAX2 is activated by
estradiol via ERα in breast cancer (7). It was also reported that PAX2 was a
common target of estrogen- and tamoxifen (ER agonist)-bound ERα and
can promote the growth of endometrial cancer cells (8). Therefore, a correlation may exist
between PAX2 and ERα in ovarian tumors, which little attention has
been paid to. In the present study, the expression of ERα was
detected in ovarian tumor types and the correlation between PAX2
and ERα was assessed.
Materials and methods
Patients and tissue samples
A total of 58 patients with ovarian serous tumor
types, including serous cystadenomas (n=30), borderline serous
cystadenomas (n=16), serous carcinomas (n=12) and patients of
ovarian mucinous tumors (n=67), including mucinous cystadenoma
(n=29), borderline mucinous cystadenoma (n=23), mucinous carcinoma
(n=15), were from the Beilun People's Hospital of Ningbo (Zhejiang,
China). Patients who received no pre-operative chemoradiation
treatment and had post-operative diagnosis as ovarian tumors were
randomly selected for the present study. All specimens were the
same as used in our previous study (9). It should be noted that one of ovarian
mucinous tumors in our previous study was not included in the
present study due to a lack of tissue. The samples were collected
and 10% neutral formalin-fixed following surgery and
paraffin-embedded following dehydration. Hematoxylin and eosin
staining and immunohistochemical analysis was used on the basis of
the availability of archived paraffin-embedded tissue blocks.
Ethical approval was obtained from the hospital and informed
consent was obtained from all patients prior to the study. Tumor
histological type was based on currently used histopathologic
criteria and the histological characteristics were reviewed by two
pathologists in a blinded manner.
Immunohistochemistry
The ERα levels were measured by immunohistochemical
analysis. Briefly, the specimens were sectioned (4 µm thick) using
a paraffin slicing machine, mounted onto poly-L-lysine-coated glass
slides and allowed to dry at 65°C for 30–60 min. The slides were
subsequently deparaffinized in xylene and transferred through three
changes of 95% ethanol. The samples were then transferred into
water. For antigen retrieval, the slides were boiled in a pressure
cooker at maximum heat for 3 min containing 0.01 mol/l sodium
citrate (pH 6.0) and cooling for 30 min at room temperature.
Endogenous peroxidase activity was inhibited in 0.3%
H2O2 for 8 min at room temperature. Following
incubation, the slides were washed three times in
phosphate-buffered saline (PBS) for 2 min. The slides were
incubated primary antibodies against rabbit anti-ERα (cat. no.
ab37438; Abcam, Cambridge, UK) at 1:100 dilution for 1 h at 37°C.
After washing three times in PBS for 2 min each, the bound primary
antibody was detected using a ready-to-use secondary antibody kit
(cat. no. K5007; Dako, Carpinteria, CA, USA) for 30 min at room
temperature and the chromogenic substrate 3,3-diaminobenzidine. The
slides were washed in distilled water, counterstained with
hematoxylin, dehydrated and mounted with permanent media.
The appropriate positive and negative controls were
included in the sections. Expression levels of ERα were evaluated
by counting at least 500 tumor cells in representative high-power
fields. Only tumor cells with nuclear staining were considered
positive for ERα. Tumor cell percentage was scored as follows: 0 =
<1% positive; 1 = ≥1 and <10% positive; 2 = 10–75% positive;
3 = >75% positive. Scoring criteria for staining intensity was
as follows: 0 = no staining; 1 =weak; 2 = moderate; 3 = strong
staining. The staining index was evaluated as the product of the
percentage of positive tumor cells and staining intensity score.
Using this method of estimation, the expression of ERα in the
tumors was evaluated by determining the staining index with scores
of 0, 1, 2, 3, 4, 6 or 9, and ‘-’ for 0 or 1, ‘+’ for 2, ‘++’ for 3
or 4, ‘+++’ for 6 or 9. In statistical analysis, ERα was considered
positive with a value of a >2.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM SPSS, Chicago, IL, USA). The χ2-test was
used to compare the expression of ERα with various histological
types. Linear regression was used to analyze the association
between PAX2 and ERα in ovarian serous tumor types. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ERα in ovarian serous
tumor types
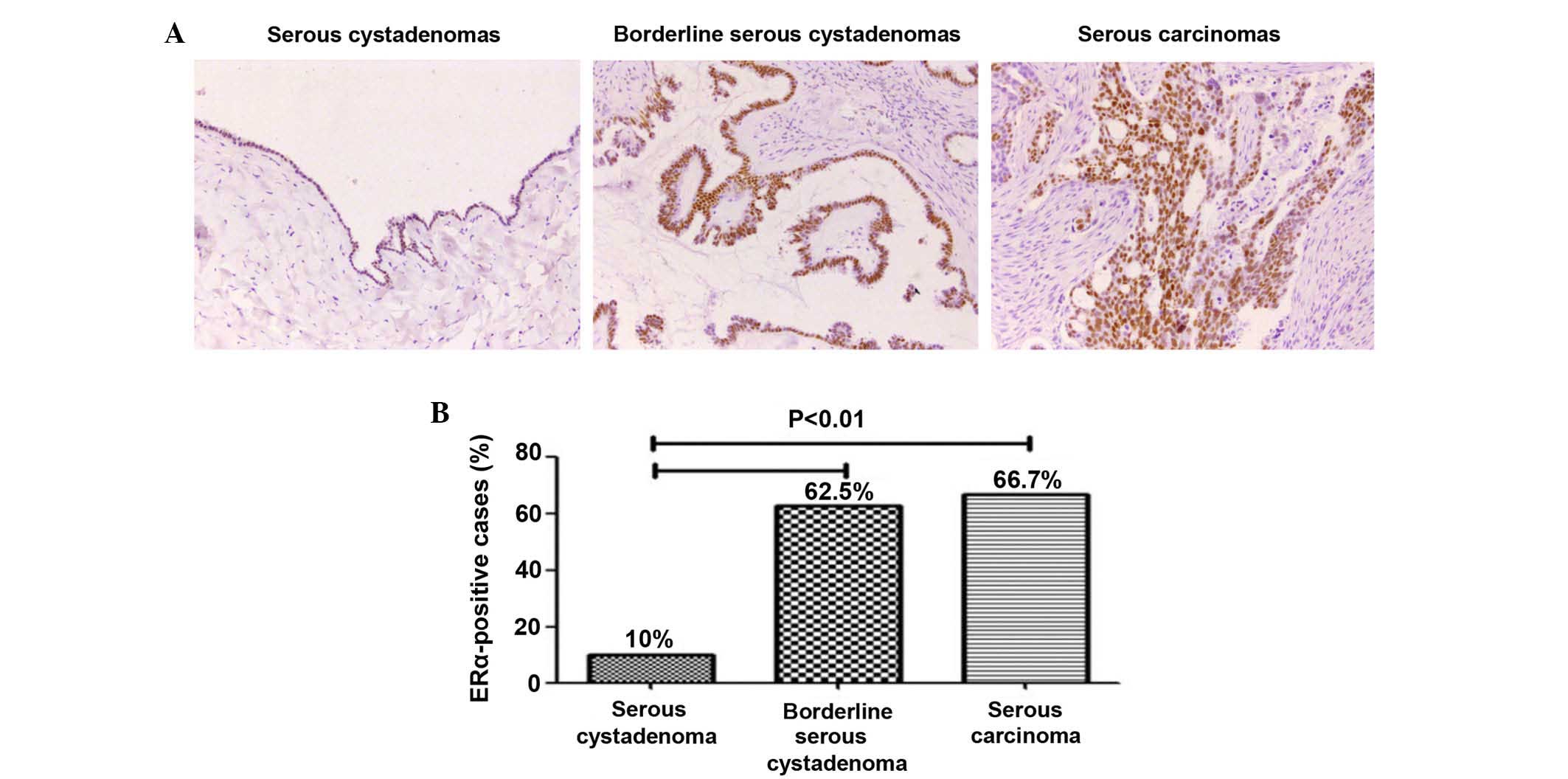
Immunohistochemical analysis revealed the expression
of ERα in ovarian serous tumors. It was shown in Fig. 1A that 10% (3/30) in serous
cystadenomas, 62.5% (10/16) in borderline serous cystadenomas and
66.7% (8/12) in serous carcinomas. The expression of ERα in
borderline serous cystadenomas and serous carcinomas were
significantly higher compared with in serous cystadenomas
(P<0.01). However, no difference between borderline serous
cystadenomas and serous carcinomas was observed (P>0.05;
Fig. 1B; Table I).
 | Table I.Expression levels of PAX2 and ERα in
ovarian serous tumors. |
Table I.
Expression levels of PAX2 and ERα in
ovarian serous tumors.
|
| PAX2 expression | ERα expression |
|---|
|
|
|
|
|---|
| Tumor type | − | + | ++ | +++ | n (%) | − | + | ++ | +++ | n (%) |
|---|
| Serous
cystadenoma | 0 | 10 | 10 | 10 | 30/30 (100) | 27 | 2 | 1 | 0 | 3/30 (10) |
| Borderline serous
cystadenoma | 0 | 0 | 5 | 11 | 16/16 (100) | 6 | 1 | 9 | 0 | 10/16 (62.5) |
| Serous carcinoma | 0 | 1 | 5 | 6 | 12/12 (100) | 4 | 2 | 5 | 1 | 8/12 (66.7) |
Expression of ERα in ovarian mucinous
tumor types
The present study additionally assessed the
expression levels of ERα in 67 cases of ovarian mucinous tumors by
immunohistochemistry. Expression of ERα was detected in 3.4% (1/29)
mucinous cystadenoma, 26.1% (6/23) borderline mucinous cystadenoma
and only 6.7% (1/15) mucinous carcinoma (Table II).
 | Table II.Expression levels of PAX2 and ERα in
ovarian mucinous tumors. |
Table II.
Expression levels of PAX2 and ERα in
ovarian mucinous tumors.
|
| PAX2 expression | ERα expression |
|---|
|
|
|
|
|---|
| Tumor type | − | + | ++ | +++ | n (%) | − | + | ++ | +++ | n (%) |
|---|
| Mucinous
cystadenoma | 29 | 0 | 0 | 0 | 0/29 (0) | 28 | 1 | 0 | 0 | 1/29 (3.4) |
| Borderline mucinous
cystadenoma | 22 | 0 | 1 | 0 | 1/23 (4) | 17 | 2 | 2 | 2 | 6/23
(26.1) |
| Mucinous
carcinoma | 15 | 0 | 0 | 0 | 0/15 (0) | 14 | 0 | 1 | 0 | 1/15 (6.7) |
Expression of PAX2 in ovarian tumor
types
In our previous study, it was demonstrated that the
expression of PAX2 was restricted to all 58 ovarian serous tumor
types (Table I). By contrast, only
one sample was positive in 68 mucinous tumors from the same
patients (Table II) (9).
Correlation between the expression
levels of PAX2 and ERα
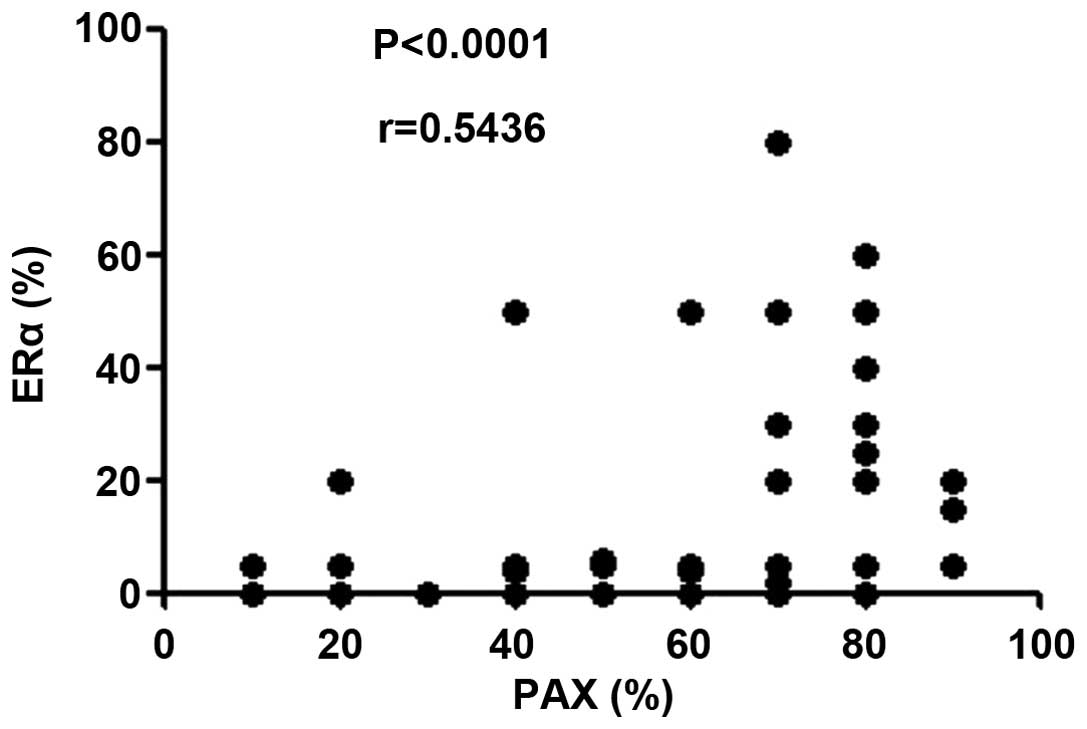
Furthermore, a correlation plot of the expression
levels of PAX2 and ERα in all 58 ovarian serous tumor samples
revealed that there is a linear correlation between them (r=0.5436;
P<0.0001; Fig. 2). This confirmed
that the expression of PAX2 is proportional to the expression of
ERα in ovarian serous tumors. By contrast, with few positive
results, no correlation was determined in ovarian mucinous tumor
samples.
Discussion
Ovarian cancer is one of the most fatal cancer types
in females, the most common histological type being serous
carcinoma. The pathogenesis of serous carcinoma remains to be
completely understood and an effective treatment strategy is
required. In the present case series, both 58 cases of ovarian
serous tumors and 67 cases of mucinous tumors consisting of three
groups, including cystadenomas and borderline cystadenomas and
carcinomas. Immunohistochemistry was performed to assess the
expression of PAX2 and ERα in ovarian serous tumors and mucinous
tumors. Our previous results revealed that PAX2 was expressed in
100% ovarian serous tumors (9). Other
researchers revealed PAX2 expression in ≥60% serous carcinomas
(10,11). The difference between the results may
be associated with the number of samples assessed. The present data
indicated that PAX2 may involved in the occurrence of ovarian
serous tumors.
No doubt estrogens are significant in the
development, growth, invasion and metastasis of breast, ovarian and
endometrial cancer. O'Donnell et al (12) identified that estrogen-driven growth
of epithelial ovarian carcinoma is mediated by the activation of
ERα-, but not ERβ-mediated transcription. Hu et al (13) found that the mRNA and protein
expression levels of ERα increased in ovarian carcinoma compared
with normal ovarian tissues. Estrogen stimulates the growth of
ovarian tumor cell lines by ERα (14). In the present study, the expression of
ERα observed in borderline cystadenomas and carcinomas were
significantly higher compared with cystadenomas in ovarian serous
tumors, which indirectly confirmed the results of previous studies
(12–14).
Previously a few results indicated that PAX2 was
activated by estradiol via ERα in breast and endometrial cancer.
Beauchemin et al (7) concluded
from their study that PAX2 may serve a novel role in the
maintenance of a low invasive behavior in luminal breast cancer
cells upon exposure to estradiol, and the overexpression and
activation of PAX2 in these cells was sufficient to reduce their
invasive ability (7). Hurtado et
al (15) suggested that endocrine
resistance in breast cancer may results due to PAX2 being a crucial
mediator of ER repression of Avian erythroblastic leukemia viral
oncogene homolog (ERBB)2 by the anti-cancer drug tamoxifen. PAX2
silencing was able to abrogate the inhibition of ERBB2
transcription and increases ERBB2-dependent cell proliferation
(15). Additionally, a previous study
determined that PAX2 was a downstream target of ERα in endometrial
cells, and PAX2 was a common target of estrogen- and tamoxifen (ER
agonist)-bound ERα and may promote the growth of endometrial cancer
cells (8).
In addition, ERα is used as a therapeutic target for
target organs of endocrine hormone. Tamoxifen is a selective
estrogen receptor modulator with anti-estrogenic activity in the
breast. As adjuvant hormone therapy, it has a clear beneficial
effect in patients with breast cancer. However, tamoxifen shows
partial estrogenic activity in the uterus and its use has been
associated with an increased incidence of endometrial cancer
(16).
However, little attention has been given to ovarian
tumor types regarding correlation between PAX2 and ERα. In the
present study of 58 ovarian serous tumor samples, a linear
correlation was observed between the expression levels of PAX2 and
ERα. It may become a potential theory basis for targeted therapy
for ovarian serous tumor types. Further research is required to
determine how PAX2 and ERα work together, and the role of targeted
therapy with tamoxifen in ovarian serous tumors.
Acknowledgements
The present study was supported by the Clinical
Research Fund Project of Zhejiang Province (no. 2013CYC-A77)
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eccles MR, He S, Legge M, Kumar R, Fox J,
Zhou C, French M and Tsai RW: PAX genes in development and disease:
The role of PAX2 in urogenital tract development. Int J Dev Biol.
46:535–544. 2002.PubMed/NCBI
|
|
3
|
Lang D, Powell SK, Plummer RS, Young KP
and Ruggeri BA: PAX genes: Roles in development, pathophysiology,
and cancer. Biochem Pharmacol. 73:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gnarra JR and Dressler GR: Expression of
Pax-2 in human renal cell carcinoma and growth nhibition by
antisense oligonucleotides. Cancer Res. 55:4092–4098.
1995.PubMed/NCBI
|
|
5
|
Silberstein GB, Dressler GR and Van Horn
K: Expression of the PAX2 oncogene in human breast cancer and its
role in progesterone-dependent mammary growth. Oncogene.
21:1009–1016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tung CS, Mok SC, Tsang YT, Zu Z, Song H,
Liu J, Deavers MT, Malpica A, Wolf JK, Lu KH, et al: PAX2
expression in low malignant potential ovarian tumors and low-grade
ovarian serous carcinomas. Mod Pathol. 22:1243–1250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beauchemin D, Lacombe C and Van Themsche
C: PAX2 is activated by estradiol in breast cancer cells of the
luminal subgroup selectively, to confer a low invasive phenotype.
Mol Cancer. 10:1482011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Chen Y, Liang J, Shi B, Wu G, Zhang
Y, Wang D, Li R, Yi X, Zhang H, et al: Hypomethylation-linked
activation of PAX2 mediates tamoxifen-stimulated endometrial
carcinogenesis. Nature. 438:981–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Ma H, Pan Y, Xiao W, Li J, Yu J
and He J: PAX2 and PAX8 reliably distinguishes ovarian serous
tumors from mucinous tumors. Appl Immunohistochem Mol Morphol.
23:280–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao FF, Krasinskas AM and Chivukula M: Is
PAX2 a reliable marker in differentiating diffuse malignant
mesotheliomas of peritoneum from serous carcinomas of müllerian
origin? Appl Immunohistochem Mol Morphol. 20:272–276. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong GX, Chiriboga L, Hamele-Bena D and
Borczuk AC: Expression of PAX2 in papillary serous carcinoma of the
ovary: Immunohistochemical evidence of fallopian tube or secondary
Müllerian system origin? Mod Pathol. 20:856–863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Donnell AJ, Macleod KG, Burns DJ, Smyth
JF and Langdon SP: Estrogen receptor-alpha mediates gene expression
changes and growth response in ovarian cancer cells exposed to
estrogen. Endocr Relat Cancer. 12:851–866. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Z, Zhu L, Tan M, Cai M, Deng L, Yu G,
Liu D, Liu J and Lin B: The expression and correlation between the
transcription factor FOXP1 and estrogen receptors in epithelial
ovarian cancer. Biochimie. 109:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cunat S, Hoffmann P and Pujol P: Estrogens
and epithelial ovarian cancer. Gynecol Oncol. 94:25–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurtado A, Holmes KA, Geistlinger TR,
Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S and
Carroll JS: Regulation of ERBB2 by oestrogen receptor-PAX2
determines response to tamoxifen. Nature. 456:663–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Machado F, Rodríguez JR, León JP,
Rodríguez JR, Parrilla JJ and Abad L: Tamoxifen and endometrial
cancer. Is screening necessary? A review of the literature. Eur J
Gynaecol Oncol. 26:257–265. 2005.PubMed/NCBI
|