Introduction
The standard surgical treatments for breast cancer
are breast-conserving surgery (BCS) and mastectomy. The essential
purpose of BCS is to completely remove the cancer while maintaining
the cosmetic appearance of the breast, which is important to the
patients. Previous studies have reported that BCS followed by
radiation had a similar outcome to that of mastectomy in terms of
mortality rate (1–5). However, a positive margin and young age
(<53–55) are significant risk factors for ipsilateral breast
tumor recurrence (IBTR) (6–8). Moreover, a meta-analysis by the Early
Breast Cancer Trialists' Collaborative Group showed that local
recurrence affected survival rate (9). Recently, Moran et al (10) reported that the American Society of
Radiation Oncology consensus guideline uses the results of a
meta-analysis of margin width and IBTR. This guideline also
indicated the optimal criteria for determining margin width in BCS.
However, despite numerous previous studies, the criteria for
intraoperative margin assessment in BCS have yet to be established.
At our institution, intraoperative specimen digital mammography
(SMMG) is used to assess the resection margins in all cases of BCS.
The aim of the present study was to evaluate the usefulness of SMMG
for achieving margin-free resection of breast tumors.
Patients and methods
Patients
A retrospective study of the medical records,
pathological diagnosis and re-evaluation by SMMG of 426 breast
cancer patients who underwent BCS at the Aichi Cancer Center
Hospital (Nagoya, Japan) between January and December, 2006 was
performed. All the patients had undergone MMG, ultrasonography (US)
and magnetic resonance imaging (MRI) preoperatively. When the
lesion was small and localized, BCS was suggested as an option to
the patient. During the study period, a total of 174 patients
underwent BCS. The surgical procedures were performed by 5
certified breast surgeons. SMMG was performed using the digital
mammography unit Senographe 2000D™ (GE Healthcare Japan Corp.,
Tokyo, Japan), which had a pixel size of 100 µm. The display
monitor had a resolution of 5 megapixels.
The study protocol was approved by our Institutional
Review Board and written informed consent was obtained from all the
patients.
Surgical procedure
BCS was performed concomitantly with sentinel lymph
node biopsy or axillary lymph node dissection in all the patients.
When scheduled, sentinel lymph node biopsy was performed at the
beginning of the surgical procedure. Preoperative localization of
the lesion was performed on the day prior to surgery by MMG, US and
MRI. For non-palpable tumors and those undetectable by US,
resection relied on hookwire-guided localization. Any localization
detected by US was marked directly on the skin. For all patients,
BCS was performed with at least a 2-cm margin around the tumor. The
pectoral fascia was resected. When the lesions were very close to
the overlying skin, the skin was also resected.
The MMG device was placed in the room adjacent to
the operating room where the resected specimen was collected and
SMMG was performed. Images were immediately captured and stored in
the hospital diagnostic imaging system. The surgeon quickly
reviewed the digital images to assess the completeness of
resection. If the resection was considered by the surgeon to be
close to the margin, a re-excision was performed intraoperatively.
The surgeon then assessed whether re-excision was complete.
Intraoperative histological margin assessment was generally not
performed.
SMMG evaluation
Two surgeons independently evaluated the SMMG
findings. The classification of the radiographic evaluation of the
resection margin was as follows: i) Negative, no cancer detected
<10 mm from the margin; ii) close, cancer detected within 5 mm
from the margin; iii) positive, cancer detected <5 mm from the
margin; and iv) lesion undetected by SMMG. The results of the two
surgeons were collectively classified as positive or negative. When
the two evaluations did not agree, the more severe evaluation was
selected (Table I).
 | Table I.Evaluation of intraoperative specimen
mammography by two clinicians. |
Table I.
Evaluation of intraoperative specimen
mammography by two clinicians.
| Clinician A | Clinician B | Final evaluation |
|---|
| Positive | Close | Positive |
| Positive | Negative | Positive |
| Positive | Undetected | Positive |
| Close | Close | Positive |
| Close | Undetected | Positive |
| Close | Negative | Negative |
| Negative | Undetected | Negative |
Histopathological evaluation
The surgical specimens were prepared for
histological analysis. The resected tissues were fixed in an
adjustable mould (11). The tissue
fixed using this method retains its polyhedral shape and
pathologists are able to evaluate all aspects of the 5-mm blocks by
hematoxylin and eosin staining. Using this procedure, all the
surfaces of the specimen may be evaluated, i.e., using the
perpendicular inked method and the tangential shaved method
(12,13). The margins were measured grossly and
microscopically by determining the presence of cancer cells at a
fixed distance from the cut edge. The distance between the margin
of the tumor and the edge of the specimen was measured by the
pathologists. A clear margin was defined as >5 mm. These
criteria also apply to ductal carcinoma in situ (DCIS).
The diagnostic reliability of SMMG was evaluated by
comparison with radiographic and histological diagnoses.
Furthermore, it was also determined whether the rate of margin
positivity was decreased by re-excision based on SMMG
evaluation.
Results
Patient characteristics
A total of 174 women who underwent BCS were
reviewed. The mean age was 54.7 years (range, 26–84 years). The
median tumor size was 1.6 cm (range, 0–4.5 cm) and 132 of the
masses were palpable (75.9%). The MMG findings revealed that 51
patients had microcalcifications (29.3%). The histopathological
diagnosis of the patients was as follows: DCIS, n=32 (18%);
invasive ductal carcinoma (IDC), n=121 (69%); invasive lobular
carcinoma, n=10 (6%); and others, n=11 (6%) (Table II).
 | Table II.Radiological and pathological patient
characteristics (n=174). |
Table II.
Radiological and pathological patient
characteristics (n=174).
| Characteristics | n (%) |
|---|
| Age (years) |
|
|
<50 | 113 (65) |
| ≥50 | 61
(35) |
| Palpability |
|
|
Non-palpable | 42
(24) |
|
Palpable | 132 (76) |
| Tumor stage |
|
| T1 | 97
(56) |
| T2 | 42
(24) |
| T3-4 | 3
(2) |
| Mammographic
image |
|
|
Calcifications only | 18
(10) |
|
Calcifications + others | 33
(19) |
|
Others | 110 (63) |
| None | 13
(8) |
| Histology |
|
| Invasive
ductual carcinoma | 121 (70) |
| Invasive
lobular carcinoma | 10
(6) |
| Ductal
carcinoma in situ | 32
(18) |
|
Others | 11
(6) |
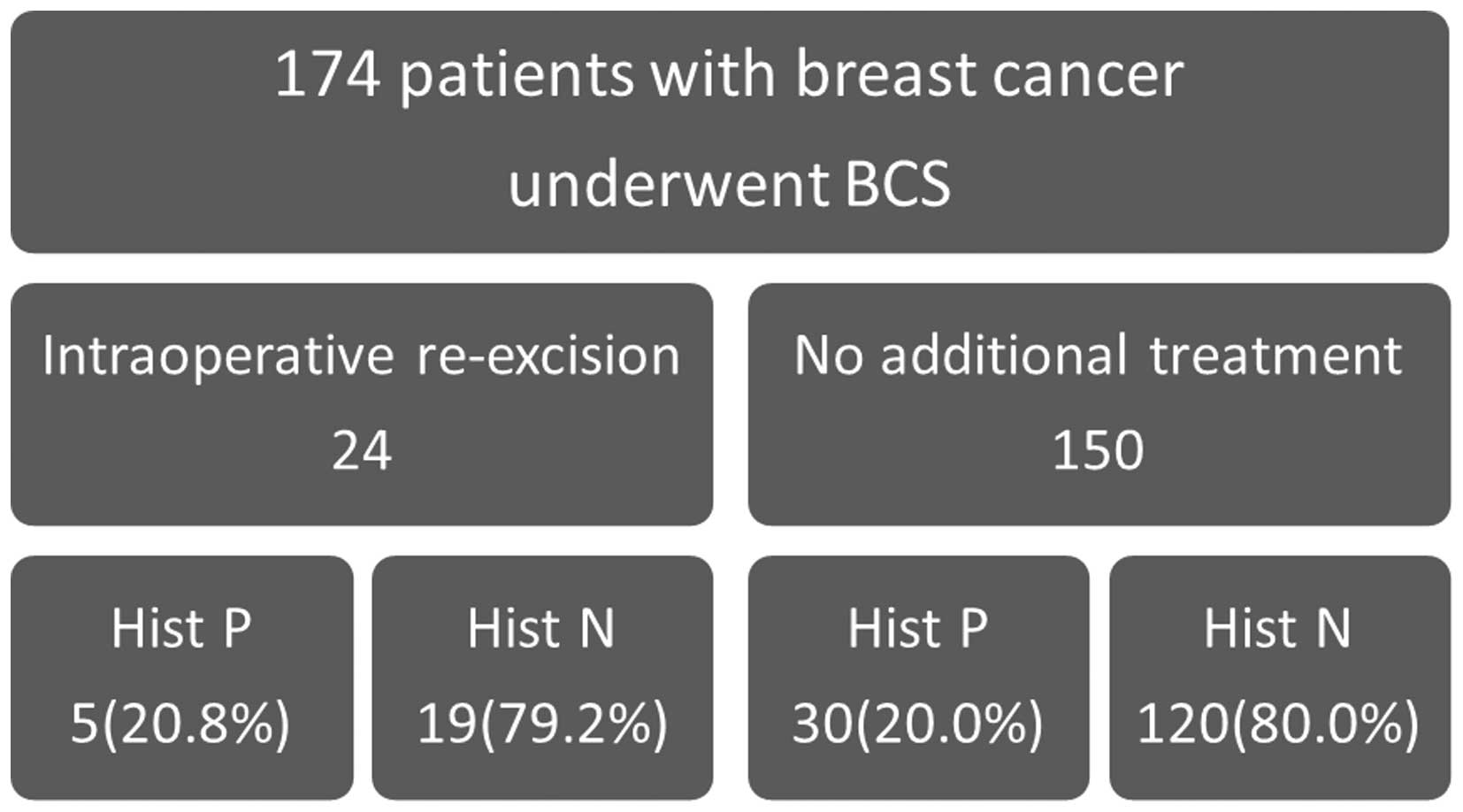
Association between histological
margin status and re-excision
The association between the histological margin
status and re-excision is shown in Fig.
1. Re-excision was performed intraoperatively in 24 cases
following positive margin identification by SMMG. Of these 24
cases, 5 (20.8%) still had positive margins following re-excision.
However, 150 cases did not undergo re-excision and 30 of those
cases had positive margins.
On radiological evaluation, 17 cases (10%) had a
positive margin, 146 cases (84%) had a negative margin and 11 cases
(6%) had undetected lesions. Histological evaluation of the margins
revealed 35 positive (20%) and 139 negative (80%) cases. The
intraoperative re-excision cases were excluded from this analysis,
as they did not undergo a second SMMG and the histological margin
status was definitively determined after the first excision.
Moreover, the lesions that were not detected by SMMG were also
excluded.
Association between SMMG and
histopathological findings
Finally, a total of 141 cases were analyzed and the
association between SMMG and histopathological findings was
evaluated (Table III). A total of
23 false-negatives and 6 false-positives were identified. The
sensitivity and specificity of SMMG margin assessment for primary
breast cancer were 20.6 and 94.6%, respectively. The positive
predictive value was 50% and the negative predictive value was
82.2%.
 | Table III.SMMG results and histological
findings. |
Table III.
SMMG results and histological
findings.
|
| Margin by histology
(n) |
|
|---|
|
|
|
|
|---|
| Margin by
imaging | Positive | Negative | Total (n) |
|---|
| Positive (n) | 6 |
6 | 12 |
| Negative (n) | 23 | 106 | 129 |
| Total (n) | 29 | 112 | 141 |
Subgroup analysis
Finally, a subgroup analysis was performed (Table IV). The number of true positive cases
was four in the microcalcification group, five in the non-palpable
group, one in group of patients aged ≥50 years and two in the IDC
group. The sensitivity and specificity for patients exhibiting
microcalcifications (n=37 after exclusion of 24 cases with
intraoperative re-excision and of nine cases in which
intra-operative SMMG did not display lesions) were 44.4 and 89.2%,
for patients aged ≥50 years they were 6.6 and 94.7%, and for
patients with non-palpable lesions they were 50 and 91.6%,
respectively. Following histopathological classification of all the
cases, the sensitivity and specificity of SMMG for IDC were 10 and
95.1%, and for DCIS they were 75 and 100%, respectively. Local
recurrence only developed in 2 cases over a median follow-up of 5.4
years; both cases had negative margins in the primary
operation.
 | Table IV.Sensitivity and specificity of each
subgroup. |
Table IV.
Sensitivity and specificity of each
subgroup.
|
| Imaging (n) | Histology (n) |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Positive | Negative | Positive | Negative | Sensitivity (%) | Specificity (%) |
|---|
| Calcifications
(n=37) | 7 | 30 | 9 | 28 | 44.4 | 89.2 |
| Non-palpable
(n=34) | 7 | 27 | 10 | 24 | 50.0 | 91.6 |
| Age ≥50 years
(n=91) | 5 | 86 | 15 | 76 | 6.6 | 94.7 |
| Histology |
|
| IDC
(n=103) | 6 | 97 | 20 | 83 | 10.0 | 95.1 |
| DCIS
(n=22) | 3 | 19 | 4 | 18 | 75.0 | 100.0 |
Discussion
When performing BCS, the main objective is to obtain
clear resection margins, as a positive margin presents a major risk
of local relapse (14,15). Intraoperative margin assessment in BCS
is crucial in order to avoid secondary surgery. The rates of
positive margins on the first attempt of BCS or lumpectomy have
been reported to be as high as 55–68% in the USA (16,17).
Despite significant improvements in breast imaging, the rate of
positive margins remains high.
SMMG is convenient, easy and cost-effective, and has
been used for margin assessment in our institution. Several studies
have demonstrated that intraoperative margin assessment using
frozen sections of the surgical specimen is useful (12,18–20).
However, histological intraoperative margin assessment may be
associated with prolonged operative time and requires pathological
expertise.
Bathla et al (21) reported that 99% of the patients
ultimately deemed as BCS candidates underwent successful breast
conservation. In that study, 14.7% of the patients underwent BCS as
the secondary surgery (21). In the
present study, only 1 patient (0.5%) underwent BCS as a second
surgical intervention, while several patients opted for mastectomy
as the second surgery. The efficacy of bidirectional SMMG was
previously reported (21,22). In the present study, however, SMMG was
not performed in two directions, as the BCS procedure involved
columnar resection between the skin and the pectoralis fascia,
rather than a spherical resection, which is common in western
countries.
Intraoperative SMMG was not proven to be useful in
the present study. There are two possible explanations: First, a
simple comparison between the pathological results and SMMG
findings was performed with the aim of determining whether the
resection margins may be reliably evaluated by SMMG. However, as
shown in Table III, the sensitivity
of SMMG was very low, particularly for patients aged ≥50 years
(6.6%). Although it was hypothesized that the density of the breast
affected the evaluation by SMMG, this hypothesis was proven to be
false by these results. In fact, in certain cases a lesion was
detected by SMMG that was not detected on preoperative MMG.
Furthermore, the majority of the cases in which a lesion was
undetectable, were those of patients aged ≥50 years. In other
subgroups, e.g. patients with microcalcifications or non-palpable
lesions, the sensitivity of SMMG was also low, whereas the
sensitivity in DCIS cases (n=22) was marginally high at 75% and the
positive predictive value was 100%. This result was also
unexpected, but it may not be reliable due to the small number of
cases. Second, the margin-positive rate in patients undergoing
re-excision was 20.8% and in those without re-excision 20.0%
(Fig. 1). Thus, the margin-positive
rate could not be reduced despite re-excision based on SMMG,
suggesting that SMMG was not useful.
Of note, in the 6 cases in which breast cancer was
diagnosed by stereotactic biopsy, the histopathological results
were in complete accord with the SMMG findings, indicating that
SMMG is likely useful in such cases.
However, the present study had certain limitations.
First, 24 cases with re-excision were excluded from the evaluation
of histology and radiology findings. The final resection margin and
histopathological results could not be compared, since not all of
the re-excision cases underwent a second SMMG. Second, there were
no exact criteria for the identification of positive margins on
radiological examination. To overcome these limitations, a
prospective study with strictly defined criteria and a standard
procedure is required to determine the usefulness of SMMG.
In conclusion, the usefulness of intraoperative SMMG
was not proven in this study. However, this procedure is likely to
be useful in selected cases, particularly those with DCIS.
References
|
1
|
Lovrics PJ, Cornacchi SD, Farrokhyar F,
Garnett A, Chen V, Franic S and Simunovic M: The relationship
between surgical factors and margin status after
breast-conservation surgery for early stage breast cancer. Am J
Surg. 197:740–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher B, Anderson S, Bryant J, et al:
Twenty-year follow-up of a randomized trial comparing total
mastectomy, lumpectomy, and lumpectomy plus irradiation for the
treatment of invasive breast cancer. N Engl J Med. 347:1233–1241.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Povoski SP, Jimenez RE, Wang WP and Xu RX:
Standardized and reproducible methodology for the comprehensive and
systematic assessment of surgical resection margins during
breast-conserving surgery for invasive breast cancer. BMC Cancer.
9:2542009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blichert-Toft M, Brincker H, Andersen JA,
et al: A Danish randomized trial comparing breast-preserving
therapy with mastectomy in mammary carcinoma. Preliminary results.
Acta Oncol. 27:671–677. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Dongen JA, Voogd AC, Fentiman IS, et
al: Long-term results of a randomized trial comparing
breast-conserving therapy with mastectomy: European Organization
for Research and Treatment of Cancer 10801 trial. J Natl Cancer
Inst. 92:1143–1150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Dongen JA, Bartelink H, Fentiman IS,
Lerut T, Mignolet F, Olthuis G, van der Schueren E, Sylvester R,
Tong D, Winter J, et al: Factors influencing local relapse and
survival and results of salvage treatment after breast-conserving
therapy in operable breast cancer: EORTC trial 10801, breast
conservation compared with mastectomy in TNM stage I and II breast
cancer. Eur J Cancer. 28A:801–805. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park CC, Mitsumori M, Nixon A, Recht A,
Connolly J, Gelman R, Silver B, Hetelekidis S, Abner A, Harris JR
and Schnitt SJ: Outcome at 8 years after breast-conserving surgery
and radiation therapy for invasive breast cancer: Influence of
margin status and systemic therapy on local recurrence. J Clin
Oncol. 18:1668–1675. 2000.PubMed/NCBI
|
|
8
|
Veronesi U, Luini A, Galimberti V and
Zurrida S: Conservation approaches for the management of stage I/II
carcinoma of the breast: Milan Cancer Institute trials. World J
Surg. 18:70–75. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moran MS, Schnitt SJ, Giuliano AE, Harris
JR, Khan SA, Horton J, Klimberg S, Chavez-MacGregor M, Freedman G,
Houssami N, et al: Society of Surgical Oncology; American Society
for Radiation Oncology: Society of Surgical Oncology-American
Society for Radiation Oncology consensus guideline on margins for
breast-conserving surgery with whole-breast irradiation in stages I
and II invasive breast cancer. J Clin Oncol. 32:1507–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ichihara S, Suzuki H, Kasami M, Aoyama H,
Sato Y, Oiwa M, Kurokawa K and Endo T: A new method of margin
evaluation in breast conservation surgery using an adjustable mould
during fixation. Histopathology. 39:85–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright MJ, Park J, Fey JV, Park A, O'Neill
A, Tan LK, Borgen PI, Cody HS III, Van Zee KJ and King TA:
Perpendicular inked versus tangential shaved margins in
breast-conserving surgery: Does the method matter? J Am Coll Surg.
204:541–549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guidi AJ, Connolly JL, Harris JR and
Schnitt SJ: The relationship between shaved margin and inked margin
status in breast excision specimens. Cancer. 79:1568–1573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houssami N, Macaskill P, Marinovich ML,
Dixon JM, Irwig L, Brennan ME and Solin LJ: Meta-analysis of the
impact of surgical margins on local recurrence in women with
early-stage invasive breast cancer treated with breast-conserving
therapy. Eur J Cancer. 46:3219–3232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singletary SE: Surgical margins in
patients with early-stage breast cancer treated with breast
conservation therapy. Am J Surg. 184:383–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christy CJ, Thorsteinsson D, Grube BJ,
Black D, Abu-Khalaf M, Chung GG, DiGiovanna MP, Miller K, Higgins
SA, Weidhaas J, et al: Preoperative chemotherapy decreases the need
for re-excision of breast cancers between 2 and 4 cm diameter. Ann
Surg Oncol. 16:697–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Sullivan MJ, Li T, Freedman G and Morrow
M: The effect of multiple reexcisions on the risk of local
recurrence after breast conserving surgery. Ann Surg Oncol.
14:3133–3140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cabioglu N, Hunt KK, Sahin AA, Kuerer HM,
Babiera GV, Singletary SE, Whitman GJ, Ross MI, Ames FC, Feig BW,
et al: Role for intraoperative margin assessment in patients
undergoing breast-conserving surgery. Ann Surg Oncol. 14:1458–1471.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sauter ER, Hoffman JP, Ottery FD,
Kowalyshyn MJ, Litwin S and Eisenberg BL: Is frozen section
analysis of reexcision lumpectomy margins worthwhile? Margin
analysis in breast reexcisions. Cancer. 73:2607–2612. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cendán JC, Coco D and Copeland EM III:
Accuracy of intraoperative frozen-section analysis of breast cancer
lumpectomy-bed margins. J Am Coll Surg. 201:194–198. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bathla L, Harris A, Davey M, Sharma P and
Silva E: High resolution intra-operative two-dimensional specimen
mammography and its impact on second operation for re-excision of
positive margins at final pathology after breast conservation
surgery. Am J Surg. 202:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fouché CJ, Tabareau F, Michenet P, Lebas P
and Simon EG: Specimen radiography assessment of surgical margins
status in subclinical breast carcinoma: A diagnostic study. J
Gynecol Obstet Biol Reprod (Paris). 40:314–322. 2011.(In French).
View Article : Google Scholar : PubMed/NCBI
|