Introduction
The second most prominent cancer in women worldwide
is cervical cancer. The general treatment for cervical cancer is
surgery, radiotherapy or both, with or without chemotherapy.
Primary concurrent chemoradiotherapy has recently been used for
advanced disease, and additionally, for early-stage locally
advanced disease (1,2). In Japan, the majority of stage IB
through to IIB disease patients are treated with radical
hysterectomy (3,4). There is a good prognosis associated with
stage IB-IIB cervical cancer; however, following surgery a
significant number of patients develop recurrence. Several
clinicopathological parameters have been used to assess the risk of
relapse, including the histological subtype, lymph node status,
lymph-vascular space involvement (LVSI), parametrial invasion and
tumor size (5–8). For patients in the high-risk groups,
postoperative radiotherapy with or without chemotherapy has been
performed previously (3,4,9,10). However, due to its impact on survival
and the quality of life, the selection of patients for adjuvant
therapy remains controversial (4,9).
Therefore, in addition to the conventional clinicopathological
parameters, the identification of more reliable and convenient
markers that are closely associated with the biological behavior of
cervical cancer and the individualization of adjuvant therapy based
on these indicators is required to improve the survival of patients
with stage I–II disease, as well as for preventing the unnecessary
use of adjuvant therapy.
The use of 18F-fluoro-2-deoxy-D-glucose
positron emission tomography (FDG-PET) with computed tomography
(CT) has been introduced over the past decade, and is now a
well-established imaging modality for the diagnosis, staging and
treatment monitoring of numerous types of cancer. Previous studies
have shown that the maximum standardized uptake value
(SUVmax), a semiquantitative simplified measurement of
the tissue deoxyglucose metabolic rate measured on FDG-PET/CT,
could be a parameter for evaluating malignancy and for assessing
the prognosis of patients with ovarian cancer (11,12) and
endometrial cancer (13–15). Therefore, the use of SUVmax
as a new biomarker that is easily measureable on PET/CT prior to
the start of treatment in patients with gynecological malignancies
has received considerable attention.
In cervical cancer, previous studies have
demonstrated the usefulness of PET/CT for the staging or assessment
of lymph node metastasis (16,17).
However, the correlation between the FDG uptake and
clinicopathological outcome of the primary tumor has not yet been
sufficiently studied and its prognostic impact remains
controversial (18–21). Furthermore, there have been few
studies regarding the clinical impact of the preoperative
SUVmax in patients with early-stage (I–II) disease
treated with radical hysterectomy (19,20,22). The
present study investigated the SUVmax of primary tumors
measured by preoperative FDG-PET/CT in stage IA2-IIB invasive
cervical cancer patients undergoing radical hysterectomy, and aimed
to clarify whether the SUVmax could be a prognostic
indicator for these patients.
Patients and methods
Patient selection
A total of 59 patients with stage IA2-IIB invasive
cervical cancer who underwent radical hysterectomy and pelvic
lymphadenectomy at Wakayama Medical University Hospital (Wakayama,
Japan) between December 2008 and June 2013 were included in this
retrospective study. All patients underwent preoperative FDG-PET/CT
scans at Wakayama Minami Radiology Clinic subsequent to providing
informed consent. No patient underwent paraaortic node
biopsy/dissection as those suspected of having paraaortic node
metastasis on preoperative PET/CT were excluded from the study. The
median age of patients was 46 years, ranging 30–68 years. The
patients were staged preoperatively according to the International
Federation of Gynecology and Obstetrics (FIGO) criteria: 6 were
stage IA2, 36 were IB1, 3 were IB2, 4 were IIA and 10 were IIB. The
postoperative pathological diagnosis and evaluation of
clinicopathological parameters, including lymph node metastasis,
LVSI and tumor size, were performed by pathologists. The
histological subtype was classified: 35 cases were squamous cell
carcinoma (SCC), 19 were adenocarcinoma (AC) and 5 were
adenosquamous carcinoma (ASC). Patients with a specific histology
other than SCC and AC/ASC were not included. The FIGO stage IB
patients with positive lymph nodes, LVSI or a larger tumor size (≥4
cm) and all FIGO stage II patients received postoperative adjuvant
therapy involving either whole pelvic irradiation with/without
chemotherapy [three courses of cisplatin (70 mg/m2) on
day 1 plus 5-fluorouracil (700 mg/m2) on days 1–4; every
4 weeks] or chemotherapy alone [three courses of paclitaxel (175
mg/m2) on day 1 plus carboplatin AUC5 on day 1; every 3
weeks]. Patients receiving primary radiotherapy/concurrent
chemoradiation therapy without surgery or receiving any form of
preoperative treatment were excluded from this study. The study was
approved by the ethics committee of Wakayama Medical
University.
FDG-PET/CT and imaging analysis
Positron emission tomography studies were performed
with a PET scanner (SET-3000BCT/L; Shimadzu, Kyoto, Japan) with an
axial resolution of 3.9 mm and a 20-cm field of view, as described
in our previous study (12). At the
time of the tracer injection, all the patients had fasted for ≥5 h
and had blood glucose levels <150 mg/dl. Images were acquired
from the top of the head to the mid-thigh 50 min after the
intravenous injection of 18F-FDG (2.6 MBq/kg body
weight). Following completion of the PET scan, CT images were
obtained with a multidetector row CT scanner (Brilliance 64;
Philips Medical Systems, Best, The Netherlands). Fusion images of
PET and CT were made using a Workstation (EV Insite; PSP Corp.,
Tokyo, Japan). FDG-PET/CT images were evaluated by a nuclear
medicine physician or radiologist. For each study, the
SUVmax of the primary tumor was measured. SUV is a
semiquantitatively analyzed value of radiotracer uptake and is
defined as the ratio of radiotracer activity per milliliter of
tissue to the activity in the injected dose corrected for decay and
the body weight of the patient.
Data analysis
The association between the SUVmax of the
primary tumor and clinicopathological or prognostic factors was
investigated. The SUVmax was compared among groups using
the Mann-Whitney U test. Receiver operating characteristic (ROC)
curve analysis was performed in order to determine the cut-off
values of the SUVmax. Overall survival (OS) was
calculated from the date of surgery to that of fatality, and
progression-free survival (PFS) was calculated from the date of
surgery to that of recurrence. The median follow-up period was 28.1
months, ranging 3.3–63 months. Survival analyses were performed
according to the Kaplan-Meier method. A comparison of the survival
between groups was performed with the log-rank test. The Cox
proportional-hazard regression model was used for multivariate
analyses to explore the impact of individual variables on survival.
P<0.05 was was considered to indicate a statistically
significant difference.
Results
Association between the
SUVmax of the primary tumor and the clinicopathological
factors
The clinicopathological characteristics and the
median SUVmax of the primary tumor in each group are
shown in Table I. The median of the
SUVmax values for all 59 patients was 4.31, with a range
of 0.00–20.29. As shown in Fig. 1A,
the SUVmax for stage IB1 was significantly higher
compared to that for stage IA2 (P=0.046), and the SUVmax
for stage IB2 was significantly higher than those for stage IA2 and
IB1 (P=0.018 and P=0.023, respectively). In addition, the
SUVmax for stage IIB was significantly higher than those
for stage IA2 (P=0.005) and IB1 (P=0.003); however, not for stage
IB2 or IIA. Similarly, the SUVmax was significantly
higher in patients with a pathologically positive pelvic lymph node
(P=0.002) (Fig. 1C) and with a
positive LVSI (P=0.044) (Fig. 1D),
while no significant correlation was observed between the
SUVmax and histological subtype (Fig. 1B). In addition, the SUVmax
in patients with a pathologically measured tumor size of ≥20 mm
(n=28) was significantly higher compared to in patients with a
tumor size of <20 mm (n=31) (data not shown).
 | Table I.Clinicopathological characteristics
of 59 cervical cancer patients. |
Table I.
Clinicopathological characteristics
of 59 cervical cancer patients.
|
Characteristics | Patients, n
(%) | Median
SUVmax |
|---|
| Total | 59 (100.0) |
4.31 |
| Stage |
|
|
|
IA2 | 6 (10.2) |
1.29 |
|
IB1 | 36 (61.0) |
3.73 |
|
IB2 | 3 (5.1) | 11.03 |
|
IIA | 4 (6.8) |
5.27 |
|
IIB | 10 (16.9) |
8.05 |
| Histology |
|
|
|
SCC | 35 (59.3) |
3.80 |
|
AC/ASC | 24 (40.7) |
4.89 |
| LN metastasis |
|
|
|
Negative | 44 (74.6) |
3.79 |
|
Positive | 15 (25.4) |
8.56 |
| LVSI |
|
|
|
Negative | 35 (59.3) |
3.81 |
|
Positive | 24 (40.7) | 7.70 |
Determination of cut-off values of the
SUVmax for predicting the presence of risk factors
As shown in Table II,
ROC curve analysis demonstrated that the optimal cut-off value of
the SUVmax for predicting a pathologically positive
lymph node status was 6.03, with a sensitivity of 80%, specificity
of 73%, and area under the curve (AUC)=0.764, while the cut-off
value of the SUVmax for predicting a positive LVSI was
4.42, with a sensitivity of 67%, specificity of 63%, and AUC=0.655.
There was a significant correlation between the SUVmax
and lymph node status (P=0.002) or LVSI (P=0.044). Similarly, ROC
curve analysis revealed that the optimal cut-off values of the
SUVmax for predicting tumor sizes of ≥20 and ≥40 mm were
4.71 and 9.66, respectively, with relatively high sensitivity and
specificity, and there was a significant correlation between the
SUVmax and tumor size.
 | Table II.Receiver operating characteristic
curve analyses of SUVmax cut-off values for predicting
risk factors. |
Table II.
Receiver operating characteristic
curve analyses of SUVmax cut-off values for predicting
risk factors.
| Variables | Sensitivity, % | Specificity, % | AUC | Optimal cut-off
SUVmax value | 95% CI | P-value |
|---|
| Positive LN
status | 80 | 73 | 0.764 | 6.03 | 0.624–0.904 | 0.002 |
| Positive LVSI | 67 | 63 | 0.655 | 4.42 | 0.512–0.799 | 0.044 |
| Tumor size, mm |
|
|
≥20 | 71 | 74 | 0.793 | 4.71 | 0.678–0.907 | <0.001 |
|
≥40 | 80 | 85 | 0.919 | 9.66 | 0.838–0.999 | 0.02 |
Correlation of the SUVmax
of the primary tumor with patient survival
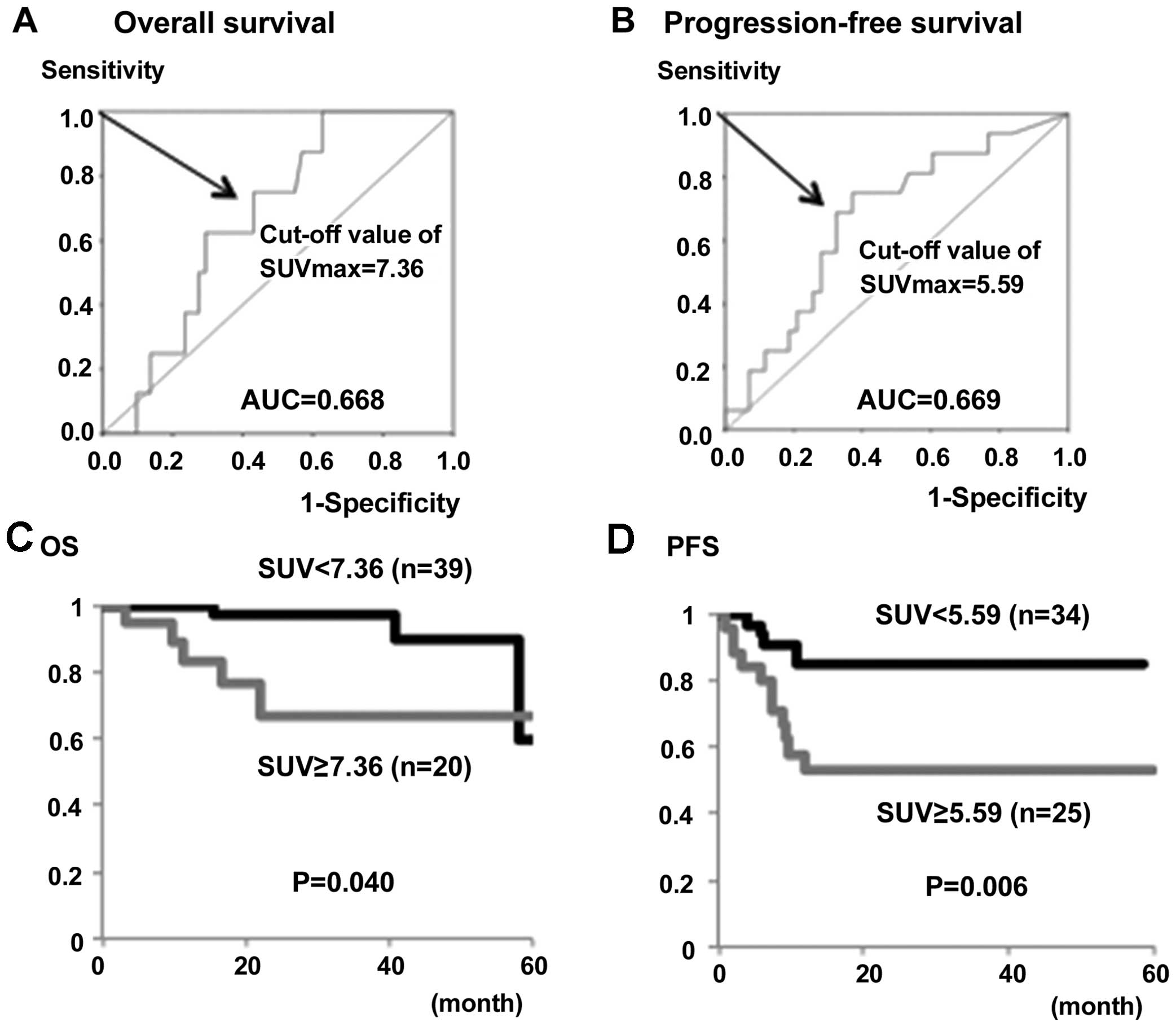
Based on the ROC curve analysis, the optimal cut-off
values of the SUVmax for predicting OS and PFS in all 59
patients were 7.36 and 5.59, respectively (Fig. 2A and B). Using these cut-off values,
the OS rate of patients with a high SUVmax (SUV ≥7.36)
was significantly lower compared with patients with a low
SUVmax(SUVmax<7.36) (P=0.04) (Fig. 2C). Similarly, the PFS rate of patients
with a high SUVmax(SUV ≥5.59) was significantly lower
compared with patients with a low
SUVmax(SUVmax<5.59) (P=0.006) (Fig. 2D).
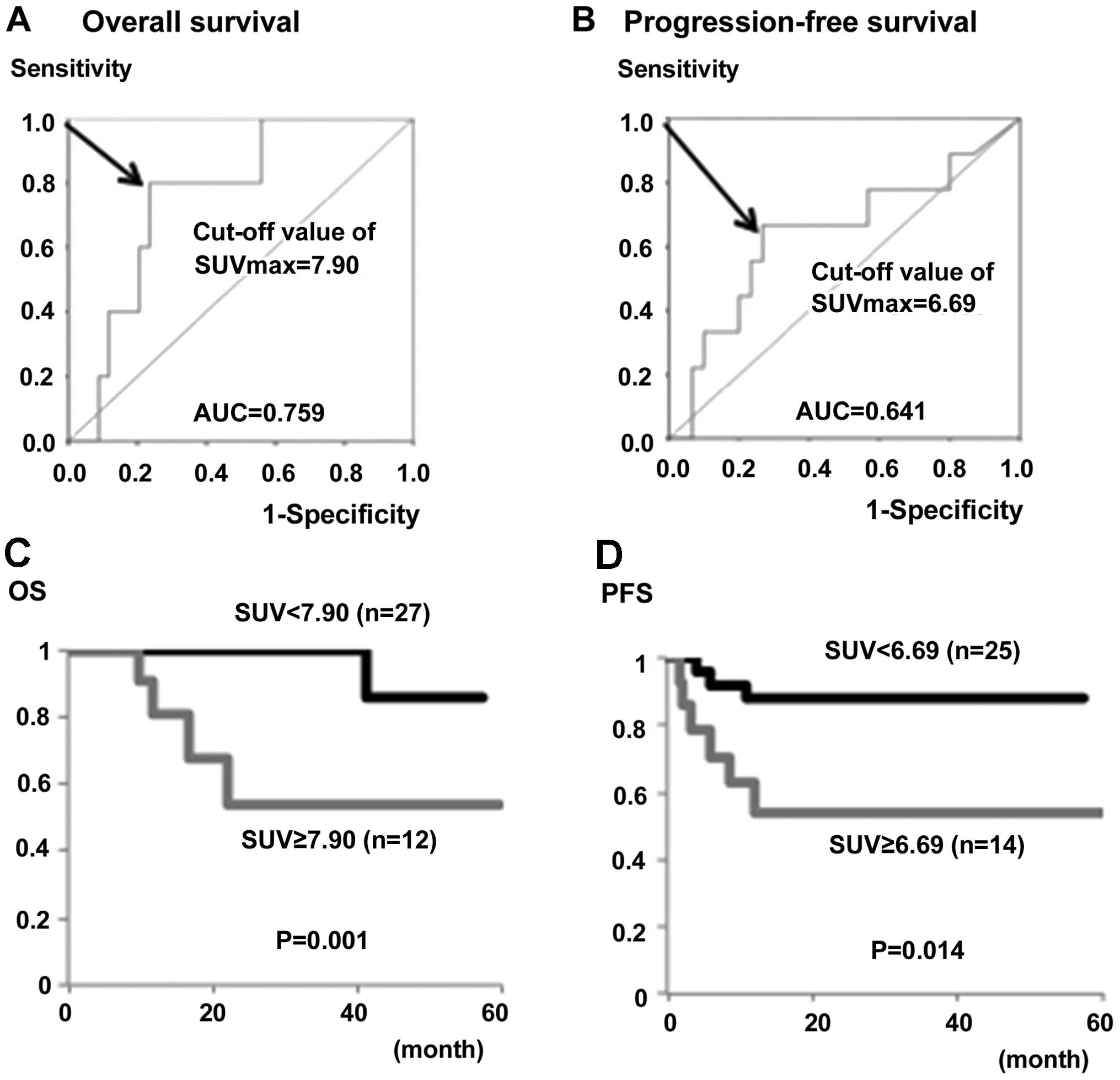
Subsequently, the impact of the preoperative
SUVmax on the prognosis of 39 patients with stage IB
disease alone was analyzed. Based on the ROC curve analysis, the
optimal cut-off values of the SUVmax for predicting OS
and PFS in stage IB patients were 7.90 and 6.69, respectively
(Fig. 3A and B). The OS and PFS rates
in patients with high SUVmax values (SUV ≥7.90 and
≥6.69) were significantly lower compared to those of patients with
low SUVmax values (P=0.001 and P=0.014, respectively)
(Fig. 3C and D).
To clarify whether the SUVmax could be an
independent prognostic factor in cervical cancer patients,
multivariate analyses were performed. As shown in Table III, multivariate analysis
demonstrated that a high SUVmax in the primary tumor was
an independent prognostic factor for impaired PFS (hazard
ratio=3.947, P=0.011) among the variables including FIGO stage,
lymph node metastasis, LVSI, tumor size and histological subtype.
Similarly, a high SUVmax was an independent factor for
predicting impaired PFS when analyzed in stage IB patients alone
(hazard ratio=4.851, P=0.026) (Table
IV).
 | Table III.Univariate and multivariate analyses
of progression-free survival in 59 cervical cancer patients. |
Table III.
Univariate and multivariate analyses
of progression-free survival in 59 cervical cancer patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | P-value | Hazard ratio | 95% CI | P-value |
|---|
| FIGO stage |
|
|
IA2-1B2 | 0.026 | 1.429 |
0.431–4.740 | 0.560 |
|
IIA-IIB |
|
| Histology |
|
|
SCC | 0.413 | 0.917 | 0.298–2.825 | 0.881 |
|
AC/ASC |
|
| LN metastasis |
|
|
Negative | 0.007 | 1.503 | 0.407–5.549 | 0.541 |
|
Positive |
|
| LVSI |
|
|
Negative | 0.030 | 1.555 | 0.470–5.143 | 0.469 |
|
Positive |
|
| Tumor size, mm |
|
|
<20 | 0.047 | 1.343 | 0.410–4.395 | 0.626 |
|
≥20 |
|
|
SUVmax |
|
|
<5.59 | 0.006 | 3.947 | 1.366–11.407 | 0.011 |
|
≥5.59 |
|
 | Table IV.Univariate and multivariate analyses
of progression-free survival in 39 stage IB patients. |
Table IV.
Univariate and multivariate analyses
of progression-free survival in 39 stage IB patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Histology |
|
|
SCC | 0.475 | 1.054 | 0.222–5.001 | 0.948 |
|
AC/ASC |
|
| LN metastasis |
|
|
Negative | 0.150 | 1.932 | 0.412–9.069 | 0.404 |
|
Positive |
|
| LVSI |
|
|
Negative | 0.380 | 1.097 | 0.276–4.363 | 0.895 |
|
Positive |
|
| Tumor size, mm |
|
|
<20 | 0.134 | 1.171 | 0.216–6.352 | 0.854 |
|
≥20 |
|
|
SUVmax |
|
|
<6.69 | 0.014 | 4.851 | 1.206–19.513 | 0.026 |
|
≥6.69 |
|
Discussion
There have been several studies showing the
association between the FDG uptake within tumors evaluated by the
SUVmax and clinical outcome in cervical cancer patients,
although its impact on disease recurrence or survival remains
controversial. Kidd et al (18) reported that the SUVmax was
a sensitive biomarker of the prognosis in patients with cervical
cancer including stage IA2-IVB treated with surgery,
chemoradiation, or palliation. Xue et al (23) also reported that the SUVmax
is predictive of the disease-free survival in stage IB1-IVB
cervical cancer patients treated with radiation therapy. By
contrast, Cho et al (20)
demonstrated that a high pretreatment SUVmax was not
predictive of recurrence in 81 patients with IB1-IVB disease
treated with surgery or concurrent chemoradiation. These different
results may be due to treatment bias as disease stages and
treatment modalities were diverse. When focusing on
surgically-treated early-stage (FIGO stage IA or IB1 to IIA)
cervical cancer, there have been controversial studies on the role
of the SUVmax (19,21,24).
Lee et al (19) and Yun et
al (24) showed that a high
SUVmax was correlated with impaired disease-free
survival, while Crivellaro et al (21) showed that the SUVmax was
not associated with recurrence. To clarify the prognostic impact of
the SUVmax on preoperative PET/CT, the present study
focused on FIGO stage IA2 to IIB patients who had undergone the
standardized surgical procedure (radical hysterectomy and pelvic
lymphadenectomy) in a single institution.
The present results showed that a high
SUVmax of the primary tumor was significantly correlated
with the presence of conventional clinicopathological risk factors,
such as positive lymph node metastasis, LVSI and a large tumor
size. In addition, the OS and PFS in patients with a higher
SUVmax were significantly lower compared with those with
a lower SUVmax. Furthermore, a high SUVmax
was an independent prognostic factor for impaired PFS on
multivariate analysis. These findings suggest that the
SUVmax of the primary tumor could be a prognostic
indicator for surgically-resected early-stage invasive cervical
cancer. Notably, the OS and PFS in patients with a higher
SUVmax were also lower when analyzed in the stage IB
group alone. As the SUVmax can be easily measured on a
preoperative FDG-PET/CT, it may be a promising non-invasive
biomarker to evaluate the risk of recurrence/fatality and to select
patients who should receive adjuvant therapy following radical
hysterectomy, particularly in stage IB patients.
In the present study, the optimal cut-off values of
the SUVmax for predicting individual risk factors and
assessing the prognosis using ROC curve analyses were determined.
The cut-off value for predicting lymph node metastasis was 6.03.
Furthermore, the cut-off levels for poor OS and PFS were 7.36 and
5.59, respectively, in all IA2-IIB patients, while those for OS and
PFS in stage IB alone were 7.90 and 6.69, respectively. These
values may be easy to use and aid the preoperative risk
stratification in each patient as an index. Consistent with the
present results, the study by Yun et al (24) showed that the cut-off value of an
SUVmax >6 was predictive of disease-free survival in
stage IA-IIA cervical cancer. By contrast, Lee et al
(19) reported that a much higher
cut-off value (SUVmax ≥13.4) was predictive of disease
recurrence in stage IB1-IIA. The study by Kidd et al
(18) showed three subgroups
according to the SUVmax cut-off values: Low (<5.2),
middle (5.2–13.3) and high risk (>13.3). The variation in the
optimal cut-off values of the SUVmax among the studies
may be dependent on the setting of PET scanning conditions and its
imaging analysis in each institution or on the targeted patient
conditions, such as disease stage.
In addition to the SUVmax, several other
metabolic parameters of FDG-PET/CT have been measured in
gynecological cancers. Kitajima et al (25) demonstrated that the metabolic tumor
volume (MTV) and total lesion glycolysis (TLG) of the primary
tumors were correlated with clinicopathological features and are
more useful for differentiating high risk from low risk compared to
the SUVmax alone in endometrial cancer. In cervical
cancer, their usefulness remains controversial. Kim et al
(22) and Chung et al
(26) reported that MTV was an
independent prognostic factor for disease recurrence in patients
with stage IA-IIB and IB-IIA, respectively. By contrast, the study
by Crivellaro et al (21)
showed that MTV and TLG were not predictors of recurrence in
IB1-IIA disease. Yoo et al (27) reported that TLG and the lymph node
status, but not MTV, were independent prognostic factors for
survival in stage IB-IVB. Considering the importance of
intratumoral FDG metabolic heterogeneity (28), the present study focusing on the
SUVmax alone is simple, but may have limitations.
Further studies using multimetabolic parameters of FDG-PET/CT,
including the SUVmax, MTV and TLG, are required to
clarify the optimal prognostic parameter for stage IA2-IIB invasive
cancer patients undergoing radical hysterectomy. Furthermore, in
combination with these metabolic parameters of FDG-PET analysis,
immunohistochemical expression of glucose-metabolism-related
proteins, such as glucose transporter 1 and cytoplasmic hexokinase
II (29,30), serum SCC antigens (31,32) and
the mean apparent diffusion coefficient on MRI (33) have also been reported to be prognostic
biomarkers. The most appropriate combination of PET parameters with
other optimal non-invasive biomarkers remains to be determined.
In conclusion, the present study demonstrated that a
high SUVmax on preoperative PET/CT correlates with an
unfavorable clinical outcome in FIGO stage IA2-IIB patients who
have undergone radical hysterectomy. These findings suggest that
the SUVmax of the primary tumor may be a promising
prognostic indicator for risk stratification in surgically-treated,
early-stage invasive cervical cancer patients.
References
|
1
|
Kesic V: Management of cervical cancer.
Eur J Surg Oncol. 32:832–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monk BJ, Tewari KS and Koh WJ:
Multimodality therapy for locally advanced cervical carcinoma:
State of the art and future directions. J Clin Oncol. 25:2952–2965.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamagami W and Aoki D: Annual report of
the committee on gynecologic oncology, the Japan society of
obstetrics and gynecology. J Obstet Gynaecol Res. 41:1861–1869.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takekuma M, Kasamatsu Y, Kado N, Kuji S,
Tanaka A, Takahashi N, Abe M and Hirashima Y: Reconsideration of
postoperative concurrent chemoradiotherapy with fluorouracil and
cisplatin for uterine cervical cancer. J Obstet Gynaecol Res.
41:1638–1643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda N, Sakuragi N, Takeda M, Okamoto K,
Kuwabara M, Negishi H, Oikawa M, Yamamoto R, Yamada H and Fujimoto
S: Multivariate analysis of histopathologic prognostic factors for
invasive cervical cancer treated with radical hysterectomy and
systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol
Scand. 81:1144–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh N and Arif S: Histopathologic
parameters of prognosis in cervical cancer-a review. Int J Gynecol
Cancer. 14:741–750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasamatsu T, Onda T, Sawada M, Kato T,
Ikeda S, Sasajima Y and Tsuda H: Radical hysterectomy for FIGO
stage I–IIB adenocarcinoma of the uterine cervix. Br J Cancer.
100:1400–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mabuchi Y, Yahata T, Kobayashi A, Tanizaki
Y, Shiro M, Ota N, Yagi S, Minami S and Ino K: Clinicopathologic
factors of cervical adenocarcinoma stages IB to IIB. Int J Gynecol
Cancer. 25:1677–1682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosa DD, Medeiros LR, Edelweiss MI,
Pohlmann PR and Stein AT: Adjuvant platinum-based chemotherapy for
early stage cervical cancer. Cochrane Database Syst Rev.
6:CD0053422012.PubMed/NCBI
|
|
10
|
Ryu HS, Chun M, Chang KH, Chang HJ and Lee
JP: Postoperative adjuvant concurrent chemoradiotherapy improves
survival rates for high-risk, early stage cervical cancer patients.
Gynecol Oncol. 96:490–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitajima K, Suzuki K, Senda M, Kita M,
Nakamoto Y, Onishi Y, Maeda T, Yoshikawa T, Ohno Y and Sugimura K:
FDG-PET/CT for diagnosis of primary ovarian cancer. Nucl Med
Commun. 32:549–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanizaki Y, Kobayashi A, Shiro M, Ota N,
Takano R, Mabuchi Y, Yagi S, Minami S, Terada M and Ino K:
Diagnostic value of preoperative SUVmax on FDG-PET/CT
for the detection of ovarian cancer. Int J Gynecol Cancer.
24:454–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitajima K, Kita M, Suzuki K, Senda M,
Nakamoto Y and Sugimura K: Prognostic significance of
SUVmax (maximum standardized uptake value) measured by
[18F]FDG PET/CT in endometrial cancer. Eur J Nucl Med Mol Imaging.
39:840–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antonsen SL, Loft A, Fisker R, Nielsen AL,
Andersen ES, Høgdall E, Tabor A, Jochumsen K, Fagö-Olsen CL,
Asmussen J, et al: SUVmax of 18FDG PET/CT as
a predictor of high-risk endometrial cancer patients. Gynecol
Oncol. 129:298–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura K, Hongo A, Kodama J and
Hiramatsu Y: The measurement of SUVmax of the primary
tumor is predictive of prognosis for patients with endometrial
cancer. Gynecol Oncol. 123:82–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gouy S, Morice P, Narducci F, Uzan C,
Gilmore J, Kolesnikov-Gauthier H, Querleu D, Haie-Meder C and
Leblanc E: Nodal-staging surgery for locally advanced cervical
cancer in the era of PET. Lancet Oncol. 13:e212–e220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kidd EA, Siegel BA, Dehdashti F, Rader JS,
Mutch DG, Powell MA and Grigsby PW: Lymph node staging by positron
emission tomography in cervical cancer: Relationship to prognosis.
J Clin Oncol. 28:2108–2113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kidd EA, Siegel BA, Dehdashti F and
Grigsby PW: The standardized uptake value for F-18
fluorodeoxyglucose is a sensitive predictive biomarker for cervical
cancer treatment response and survival. Cancer. 110:1738–1744.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YY, Choi CH, Kim CJ, Kang H, Kim TJ,
Lee JW, Lee JH, Bae DS and Kim BG: The prognostic significance of
the SUVmax (maximum standardized uptake value for F-18
fluorodeoxyglucose) of the cervical tumor in PET imaging for early
cervical cancer: Preliminary results. Gynecol Oncol. 115:65–68.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho SH, Lim JY, Kim SN, Hong S, Chung HW,
So Y, Kim WY and Lee SJ: The prognostic significance of
pretreatment [18F]FDG-PET/CT imaging in patients with uterine
cervical cancer: Preliminary results. Eur J Gynaecol Oncol.
36:30–35. 2015.PubMed/NCBI
|
|
21
|
Crivellaro C, Signorelli M, Guerra L, De
Ponti E, Buda A, Dolci C, Pirovano C, Todde S, Fruscio R and Messa
C: 18F-FDG PET/CT can predict nodal metastases but not
recurrence in early stage uterine cervical cancer. Gynecol Oncol.
127:131–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim BS, Kim IJ, Kim SJ, Nam HY, Pak KJ,
Kim K and Yun MS: The prognostic value of the metabolic tumor
volume in FIGO stage IA to IIB cervical cancer for tumor
recurrence: Measured by F-18 FDG PET/CT. Nucl Med Mol Imaging.
45:36–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue F, Lin LL, Dehdashti F, Miller TR,
Siegel BA and Grigsby PW: F-18 fluorodeoxyglucose uptake in primary
cervical cancer as an indicator of prognosis after radiation
therapy. Gynecol Oncol. 101:147–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yun MS, Kim SJ, Pak K and Lee CH:
Additional prognostic value of SUVmax measured by F-18
FDG PET/CT over biological marker expressions in surgically
resected cervical cancer patients. Oncol Res Treat. 38:413–416.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitajima K, Suenaga Y, Ueno Y, Maeda T,
Ebina Y, Yamada H, Okunaga T, Kubo K, Sofue K, Kanda T, et al:
Preoperative risk stratification using metabolic parameters of
(18)F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl
Med Mol Imaging. 42:1268–1275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung HH, Kim JW, Han KH, Eo JS, Kang KW,
Park NH, Song YS, Chung JK and Kang SB: Prognostic value of
metabolic tumor volume measured by FDG-PET/CT in patients with
cervical cancer. Gynecol Oncol. 120:270–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo J, Choi JY, Moon SH, Bae DS, Park SB,
Choe YS, Lee KH and Kim BT: Prognostic significance of volume-based
metabolic parameters in uterine cervical cancer determined using
18F-fluorodeoxyglucose positron emission tomography. Int
J Gynecol Cancer. 22:1226–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kidd EA and Grigsby PW: Intratumoral
metabolic heterogeneity of cervical cancer. Clin Cancer Res.
14:5236–5241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong SY, Lee JM, Ki KD, Choi YJ, Seol HJ,
Lee SK, Huh CY, Kim GY and Lim SJ: Correlation between FDG uptake
by PET/CT and the expressions of glucose transporter type 1 and
hexokinase II in cervical cancer. Int J Gynecol Cancer. 22:654–658.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SI, Suh DS, Kim SJ, Choi KU and Yoon
MS: Correlation between biological marker expression and
F-fluorodeoxyglucose uptake in cervical cancer measured by positron
emission tomography. Onkologie. 36:169–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura K, Okumura Y, Kodama J, Hongo A,
Kanazawa S and Hiramatsu Y: The predictive value of measurement of
SUVmax and SCC-antigen in patients with pretreatment of
primary squamous cell carcinoma of cervix. Gynecol Oncol.
119:81–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan L, Cheng J, Zhou M, Yao Z and Zhang Y:
The SUVmax (maximum standardized uptake value for F-18
fluorodeoxyglucose) and serum squamous cell carcinoma antigen
(SCC-ag) function as prognostic biomarkers in patients with primary
cervical cancer. J Cancer Res Clin Oncol. 138:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miccò M, Vargas HA, Burger IA, Kollmeier
MA, Goldman DA, Park KJ, Abu-Rustum NR, Hricak H and Sala E:
Combined pre-treatment MRI and 18F-FDG PET/CT parameters
as prognostic biomarkers in patients with cervical cancer. Eur J
Radiol. 83:1169–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|