Introduction
Non-small-cell lung cancer (NSCLC) is a
life-threatening malignancy, exhibiting the highest incidence and
mortality rate amongst all types of cancer worldwide (1). Chemotherapy is traditionally the
mainstay of treatment for advanced NSCLC, with a median survival of
~10 months (2), while the emergence
of targeted therapy has not only prolonged patient survival, but
also significantly improved the patients' quality of life.
Gefitinib and erlotinib, the first oral tyrosine kinase inhibitors
(TKIs), have led to a significant improvement in the outcome of
patients with epidermal growth factor receptor (EGFR) mutations,
with a median survival of ~20 months (3,4), as
demonstrated in previous clinical studies (5–7). Icotinib,
a novel EGFR-TKI, exerted a distinctly inhibitory effect on NSCLC
in vivo and in vitro. A phase III clinical study
(ICOGEN) demonstrated that icotinib exhibits high efficacy in the
re-treatment of advanced NSCLC, compared with gefitinib (8). The efficacy of small-molecule TKIs has
been shown to be associated with the EGFR mutation status. Exons
18–21 are the most common mutation sites in EGFR, with exons 19 and
21 being sensitive to targeted drug therapies. According to the
subgroup analysis of recent phase III studies, patients with exon
19 deletion who received elotinib exhibited a better response
compared with those with exon 21 L858R mutation (9). Furthermore, it was demonstrated by a
previous retrospective study that the efficacy of gefitinib in
patients harboring exon 19 deletion was comparable to that in
patients harboring exon 21 L858R mutation (10). We retrospectively analyzed the
post-treatment survival data of patients who were treated with
icotinib, with the aim of further elucidating the association
between the two most common EGFR mutations (exon 19 deletion and
exon 21 L858R mutation) and the efficacy of icotinib.
Patients and methods
Patient inclusion criteria
After reviewing the clinical data of stage IIIb and
IV NSCLC patients with complete follow-up records, who were
admitted to the Affiliated Hospital of Qingdao University Medical
College (Qingdao, China) between August, 2012 and August, 2014, 104
patients with EGFR mutations who received icotinib were identified.
The inclusion criteria were as follows: (i) Patients with EGFR exon
19 deletion or exon 21 L858R mutation; (ii) at least 1 measurable
clinical lesion according to Response Evaluation Criteria in Solid
Tumors (RECIST), version 1.1 (11);
(iii) patients who did not receive prior treatment with other TKIs;
and (iv) an Eastern Cooperative Oncology Group performance status
score of ≤3.
Evaluation of efficacy and toxicity of
icotinib
Efficacy evaluation was performed 1 month after the
administration of icotinib. Patients with response to treatment or
stable disease (SD) underwent computed tomography and other imaging
tests every 2 months, the results of which were used for efficacy
evaluation. The treatment response was assessed with RECIST 1.1 as
follows: Complete response (CR), partial response (PR), SD and
progressive disease. The response rate (RR) included CR and PR. The
time to response was calculated from the date of initiation of
icotinib administration to the date when a clinical response was
detected.
The National Cancer Institute Common Toxicity
Criteria, version 3 (http://ctep.cancer.gov), were used to evaluate
toxicity and grade 0–4 side effects.
Detection of EGFR mutations
Pyrosequencing was used to assess the EGFR mutation
status of patients by collecting 82 biopsy samples and 22 tissue
samples. As one of the major EGFR detection methods currently used,
pyrosequencing has become the most widely used detection method in
clinical practice due to its specificity. The patients' DNA was
re-tested by using ADx EGFR Mutations Detection kit (Amoy
Diagnostics, Xiamen, China), which recently received State Food and
Drug Administration approval for clinical application in mainland
China. The kit used the principle of Amplified Refractory Mutation
System and covered the 29 EGFR mutation hotspots from exon 18 to
21. The assay was carried out according to the manufacturer's
protocol with the MX3000P quantitative polymerase chain reaction
system (Stratagene, La Jolla, CA, USA). The results were determined
as positive or negative according to the criteria defined by the
manufacturer's instructions. The results of ADx-AMRS were compared
with those of direct sequencing. BioAsia (Shanghai, China) examined
all the samples used in this study and provided the detection
reports.
Follow-up
Information on the progression-free survival (PFS)
of the patients was obtained through outpatient visits or telephone
follow-up.
Follow-up results were available for all 104
patients. The last follow-up visit was conducted on March 1st,
2015. PFS was defined as the time from the initiation of icotinib
administration to treatment failure (death, progressive disease or
intolerable toxicity), or the last visit.
Statistical analysis
All statistical analyses were performed using SPSS
software for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA).
The Fisher's exact test was used to compare baseline
characteristics and the RR of patients with different EGFR mutation
types. The Kaplan-Meier method was used to draw survival curves and
the PFS data between treatment groups were compared using the
log-rank test. P<0.05 was considered to indicate statistically
significant differences.
Results
General characteristics of the
patients
A total of 104 patients diagnosed with NSCLC
harboring EGFR exon 19 deletion or exon 21 L858R mutation between
August, 2012 and August, 2014, were enrolled in this study. Of the
104 patients, 49 were male and 55 were female. The median age of
all the patients was 62 years. According to the pathological
diagnosis, 101 patients were confirmed with adenocarcinoma and 3
had other types of NSCLC. A total of 10 patients were at stage IIIb
and the remaining patients at stage IV; among these, 20 patients
had recurrent and metastatic NSCLC. Of the 104 patients, 60
harbored an exon 19 deletion and 44 a 21 L858R mutation. The
general characteristics of the patients are summarized in Table I. There were significant differences
between the NSCLC patients harboring exon 19 deletion and those
harboring the L858R point mutation in the distribution of the
categorical characteristics, i.e., male predominance (48.3 vs.
45.5%, P=0.843) and poor PS (36.7 vs. 32.5%, P=0.831).
 | Table I.Patient characteristics (n=104). |
Table I.
Patient characteristics (n=104).
| Characteristics | Patients, no.
(%) |
|---|
| Gender |
|
| Male | 49 (47.0) |
|
Female | 55 (53.0) |
| Median age, years
(range) | 62
(42–75) |
| ECOG PS |
|
| 0 or
1 | 67 (64.0) |
| 2 or
3 | 37 (36.0) |
| Histology |
|
|
Adenocarcinoma | 101 (97.0) |
| Not
otherwise specified | 3 (3.0) |
| EGFR mutation |
|
| Exon 19
deletion | 60 (58.0) |
| L858R
mutation | 44 (42.0) |
| Disease stage |
|
| IIIB | 10 (9.6) |
| IV | 84 (80.8) |
|
Recurrence | 10 (9.6) |
| Number of prior
regimens |
|
| 0 | 65 (62.5) |
| ≥1 | 39 (37.5) |
Clinical efficacy evaluation
Of the 104 patients, an objective response (PR+CR)
was obtained in 64, including a CR in 2 patients, representing an
RR of 61.5%. The patients achieving CR+PR included 38 with exon 19
deletion and 26 with exon 21 L858R mutation, with objective RRs of
63.3 and 59%, respectively. Table II
shows the overall RR of the two groups. There was no significant
difference in RR between the two groups (P=0.688).
 | Table II.Response to icotinib according to EGFR
genotype. |
Table II.
Response to icotinib according to EGFR
genotype.
| Type of mutation | Responders, n | RR, % | P-valuea |
|---|
| Exon 19 deletion
(n=60) | 38 | 63.3 | 0.688 |
| L858R mutation
(n=44) | 26 | 59.1 |
|
| Total (n=104) | 64 | 61.5 |
|
Correlation of the EGFR genotype with
PFS
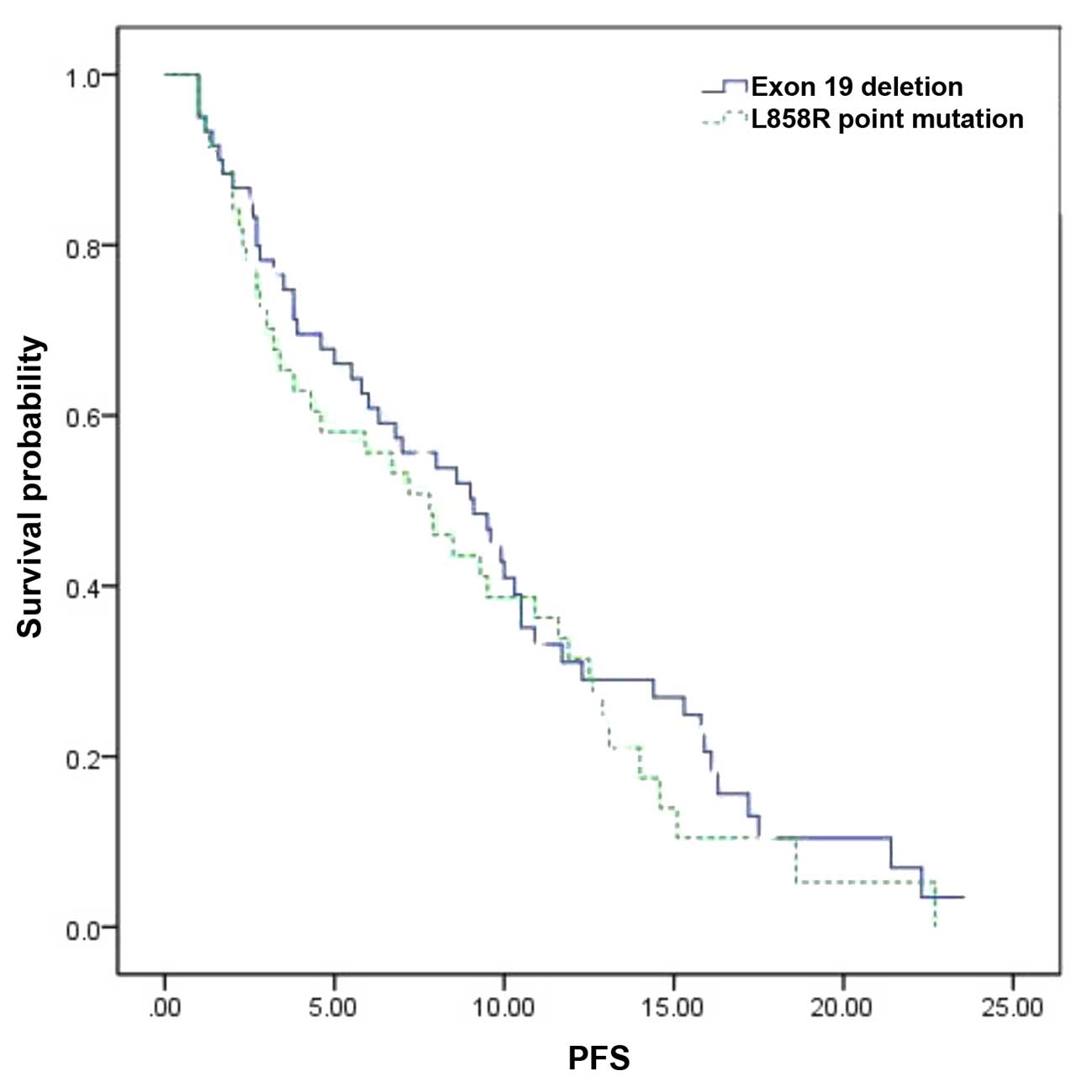
The median PFS in the overall patient population was
8.5 months [95% confidence interval (CI): 7.8–10.4 months]. With
regard to the EGFR genotype, the median PFS was 9.1 months (95% CI:
7.8–11.3 months) in patients with exon 19 deletion and 7.8 months
(95% CI: 6.5–10.4 months) in those with exon 21 L858R mutation.
There was no statistically significant difference in PFS between
the two genotypes (P=0.423, Fig. 1,
Table III).
 | Table III.PFS according to the EGFR
genotype. |
Table III.
PFS according to the EGFR
genotype.
| Type of mutation | n | PFS (months) | 95% CI |
|---|
| Exon 19 deletion | 60 | 9.1 | 7.8–11.3 |
| L858R mutation | 44 | 7.8 | 6.5–10.4 |
Toxic and adverse events
The main toxic and adverse events associated with
the administration of icotinib were a rash in 41/104 (39.4%) and
diarrhea in 27/104 patients (25.9%). Grade III–IV toxic and adverse
events were rarely encountered, with an overall incidence of 10.5%
(11/104) in all patients. Among these, grade III rash was observed
in 6 patients, grade III diarrhea in 4 and grade III fatigue in 1
patient; no patients reported grade IV adverse reactions.
Discussion
Icotinib is the most commonly used EGFR-TKI in China
and the third worldwide after erlotinib and gefitinib. According to
the ICOGEN clinical study, icotinib has a similar efficacy to
gefitinib (12,13) in the re-treatment of advanced NSCLC
and is associated with a significantly lower incidence of adverse
events (8,14). Icotinib is currently the standard
therapy for advanced NSCLC in China. The median PFS was 10.8 months
in this study, indicating that icotinib is effective in the
treatment of patients with EGFR mutations. There was no difference
in PFS between patients harboring exon 19 deletion and those with
exon 21 L858R mutation. The overall survival (OS) data in this
study were inconclusive.
Exon 19 deletion and exon 21 L858R mutation are the
most common types of EGFR mutation. A European phase III randomized
controlled trial of erlotinib (Tarceva®) vs.
chemotherapy (EURTAC trial) demonstrated that NSCLC patients with
exon 19 deletion have a more favorable response rate, PFS and OS
compared with patients with exon 21 L858R mutation (9). Based on in vitro cell cultures,
another study demonstrated that exon 19 deletion may have a greater
affinity for TKIs and display a higher sensitivity to treatment
compared with exon 21 L858R mutation (15). However, a retrospective clinical study
by Igawa et al (10) revealed
no significant differences in RR, PFR or OS between patients with
exon 19 deletion and those with exon 21 L858R mutation after
receiving gefitinib, which is consistent with the stratification
analysis results of another two large, randomized, phase III trials
[NEJ002 (12) and WJTOG3405 (13)]. Hence, there remain disputes regarding
the correlation between EGFR mutation sites and therapeutic
response. Icotinib, a novel targeted agent based on structural
modification of erlotinib, differs from gefitinib and erlotinib in
its metabolism. In our study, we observed that there were no
significant differences in RR and PFS between the two groups and
that icotinib imparted a PFS benefit in patients with exon 19
deletion compared with those with exon 21 mutation. Larger samples
and a prolonged study duration are required to determine whether
there are significant differences in PFS and OS between the two
groups treated with icotinib. Further studies are required to
determine whether the different responses between the two groups
are associated with the mutation or drug configuration.
This study, which conducted a retrospective survival
analysis, also has several limitations. First, it was a
retrospective study that may contain selection bias. For example,
there were minor differences between our study and previous
clinical studies in terms of mean age, proportion of females and
proportion of adenocarcinoma patients. However, no major
differences were found in patient characteristics, e.g., gender and
PS score (determined by the Fisher's exact test). Second,
gefitinib, erlotinib and icotinib differ in their molecular
structure. It was previously demonstrated that gefitinib and
erlotinib exhibit different efficacy in exon 19 deletion and exon
21 L858R mutation regarding patient outcomes; thus, differences in
molecular structures may affect efficacy. Third, there may be no
statistical difference in PFS benefit due to the small sample size;
the OS results were inconclusive due to the short follow-up
duration and the PFS data were censored. These factors may affect
the results of this study.
In conclusion, NSCLC patients with exon 19 deletion
in the EGFR gene tended to have a better PFS compared with those
with exon 21 L858R mutation following treatment with icotinib.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, et al: Erlotinib in previously treated non-small-cell
lung cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thatcher N, Chang A, Parikh P, Rodrigues
Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell lung cancer: Results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Shi Y, Zhang L, et al: A
randomized, double-blind phase III study of icotinib versus
gefitinib in patients with advanced non-small cell lung cancer
(NSCLC) previously treated with chemotherapy (ICOGEN). J Clin
Oncol. 29(Suppl): S75222011.
|
|
9
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Igawa S, Kasajima M, Ishihara M, Kimura M,
Hiyoshi Y, Asakuma M, Otani S, Katono K, Sasaki J and Masuda N:
Comparison of the efficacy of gefitinib in patients with non-small
cell lung cancer according to the type of epidermal growth factor
receptor. Oncology. 87:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu A, Shi C, Xiong L, Chu T, Pei J and Han
B: Efficacy and safety evaluation of icotinib in patients with
advanced non-small cell lung cancer. Chin J Cancer Res. 25:90–94.
2013.PubMed/NCBI
|
|
15
|
Carey KD, Garton AJ, Romero MS, Kahler J,
Thomson S, Ross S, Park F, Haley JD, Gibson N and Sliwkowski MX:
Kinetic analysis of epidermal growth factor receptor somatic mutant
proteins shows increased sensitivity to the epidermal growth factor
receptor tyrosine kinase inhibitor, erlotinib. Cancer Res.
66:8163–8171. 2006. View Article : Google Scholar : PubMed/NCBI
|