Introduction
Neutrophils belong to the phagocyte system and
represent the first cellular components of the inflammatory
response and key components of innate immunity (1). Chemotherapy-induced neutropenia (CIN) is
the most serious hematological toxicity of cancer chemotherapy
(2). CIN is associated with the risk
of life-threatening infections, as neutropenia blunts the
inflammatory response, allowing bacterial multiplication and
invasion (3). In neutropenic
patients, infection may occur with minimal signs and symptoms and
may rapidly progress to sepsis with multi-organ failure (4). As fever may constitute the only sign in
these patients, febrile neutropenia (FN) should be considered a
true emergency (5).
FN is the most frequent complication and the leading
cause of morbidity and mortality in oncology patients undergoing
intensive chemotherapy; it is also associated with a significant
economic and social burden on the health system (6). Early recognition of FN and initiation of
broad spectrum empirical systemic antibacterial therapy is crucial
for avoiding progression to sepsis and possible death (7). CIN may also necessitate chemotherapy
dose reductions, delays or even discontinuation, which may
compromise treatment outcome (8).
Recently, high-dose chemotherapy has been performed
more often for malignant diseases, such as leukemia (9); this is partly due to improved patient
care, and partly due to the advances in the methods for preventing
and handling adverse effects (2,10).
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that
mobilizes CD34 stem cells, increases neutrophil production and
stimulates neutrophil function. Following myelotoxic chemotherapy,
recombinant human G-CSF mobilizes progenitor cells from the bone
marrow into the peripheral circulation and, thus, is used to
prevent neutropenia (11). European
recommendations stipulate that prophylactic G-CSF use may reduce
treatment-related morbidity and the duration of the treatment
protocol, by reducing the proportion of patients with FN (12). A meta-analysis concluded that primary
prophylaxis reduced the incidence of FN in patients receiving
chemotherapy for solid tumors and lymphoma (10). Despite these benefits, however, G-CSF
is not administered to all patients receiving systemic chemotherapy
due to the unaffordable cost associated with its routine use. The
selective use of G-CSF in patients at high risk for CIN and its
complications may be more cost-effective (13).
Several studies in adults have sought to identify
risk factors that may predispose patients to CIN and its
consequences (3,14,15) in an
attempt to develop a predictive model capable of identifying
patients at greater risk and provide clear guidelines to use
expensive preventive strategies more cost-effectively (11); however, similar trials in pediatrics
are sparse. The aim of the present study was to determine the risk
factors associated with CIN and its consequences in pediatric
patients undergoing systemic chemotherapy in order to apply
appropriate preventive strategies.
Patients and methods
Patients
Data for this prospective cohort study were
collected from 50 pediatric cancer patients who presented with 113
episodes of neutropenia as a consequence of systemic
myelosuppressive therapy. The patients were admitted to the
Pediatric Oncology Unit of the Zagazig University Children's
Hospital (Zagazig, Egypt) in the period from the 1st of June, 2013
to the 1st of June, 2014. All the patients were subjected to full
medical history taking, thorough clinical examination, routine
investigations and management according to our standard
institutional guidelines (16).
Methods
Risk factors associated with CIN were classified as
patient-specific (age, gender and anthropometric measurements),
disease-specific (tumor type and stage) and regimen-specific
(phase/cycle, drug used and dosage).
The consequences of CIN, namely FN, systemic and/or
local infections, dose modifications (reduction, delay or
discontinuation of chemotherapy, in-hospital stay and total medical
costs for the treatment of neutropenic episodes were evaluated and
analyzed.
CIN was defined as an absolute neutrophil count
(ANC; polymorphonuclear and band forms) <0.5×109/l,
or 1.0×109/l and expected to decrease, or a leukocyte
count <1.0×109/l (1).
FN was defined as single oral temperature
measurement of ≥38.5, or 3 measurements of ≥38 within a 24-h
period, taken at least 4 h apart (17).
Ethics
This study was conducted in accordance with the
ethical standards and approved by the Institutional Review Board of
the Faculty of Medicine, Zagazig University. Written informed
consent was obtained from each patient or guardian prior to
enrollment in the study.
Statistical analysis
Data were prospectively tabulated and analyzed using
the SPSS software, version 16 (SPSS Inc., Chicago, IL, USA).
Unpaired t-test, analysis of variance (ANOVA), Chi-square and
Pearson's correlation coefficient were used as appropriate. P-value
<0.05 was considered to indicate statistically significant
differences.
Results
Patient characteristics
A total of 50 patients, who presented with 113
episodes of CIN during the study period, were enrolled in this
study. Their mean age was 5.6±2.8 years (range, 10 months-13 years)
and 58/113 (51.3%) of the patients were male. Acute lymphoblastic
leukemia (ALL) of different subtypes was the leading primary
diagnosis among our cases, whereas other hematological malignancies
and solid tumors exhibited significantly lower prevalence rates
(Fig. 1).
Neutropenic episodes
A description of the neutropenic episodes is
presented in Table I. Among our
patients, 28% presented with significant neutropenia for the first
time, while 72% had recurrent episodes throughout the treatment
course. The mean ANC was 225.5±128.5 ×109/l (range,
10–497 ×109/l) starting at 14.2±16.3 days (range, 2–100
days) after the onset of chemotherapy and resolved within 11.2±7.3
days, either with (45.1%) or without (54.9%) G-CSF.
 | Table I.Description of neutropenia episodes
(n=113). |
Table I.
Description of neutropenia episodes
(n=113).
| Variables | Values |
|---|
| Age, years |
|
| Mean ±
SD | 5.61±2.82 |
| Gender |
|
| Male, n
(%) | 58 (51.3) |
| Female,
n (%) | 55 (48.7) |
| Pre-treatment TLC,
×109/l |
|
| Mean ±
SD | 14.300±3.810 |
|
Range | 1.300±23.000 |
| Previous
neutropenias, n (%) |
|
| No | 32 (28.3) |
|
Yes | 81 (71.1) |
| Number of previous
attacks |
|
| Mean ±
SD | 3.3±1.95 |
|
Range | 1–9 |
| TLC during
neutropenia, ×109/l |
|
| Mean ±
SD | 1.450±5.480 |
|
Range | 1.000–10.000 |
| ANC during
neutropenia, ×109/l |
|
| Mean ±
SD | 225.0±128.3 |
|
Range | 10–497 |
| Onset after
chemotherapy, days |
|
| Mean ±
SD | 14.2±16.3 |
|
Range | 2–100 |
| Duration of
neutropenia, days |
|
| Mean ±
SD | 11.2±7.3 |
|
Range | 2–42 |
| Recovery, n
(%) |
|
| With
G-CSF | 51 (45.1) |
| Without
G-CSF | 62 (54.9) |
| Duration of G-CSF,
days |
|
| Mean ±
SD | 4.98±3.1 |
|
Range | 1–18 |
ANC correlation with patient-,
disease- and regimen-specific characteristics
Analysis of our data revealed no significant
correlation between ANC and any of the patient-specific
characteristics, such as age (r=0.16), or anthropometric
measurements (weight, r=0.14; height, r=0.15; body mass index,
r=0.02; P>0.05). Moreover, no statistically significant
difference in ANC was detected between different genders (male vs.
female, 216.3±140.3 vs. 234.5±114.8, respectively; P=0.47). One-way
ANOVA for comparing ANC in different underlying diseases revealed a
significantly lower ANC in B-ALL, neuroblastoma, Burkitt's lymphoma
and lymphoblastic lymphoma, B-immunophenotype (Fig. 2). However, no significant correlation
was found between ANC and different disease stage (r=−0.15) or
patient risk (r=−0.03). A significant inverse correlation between
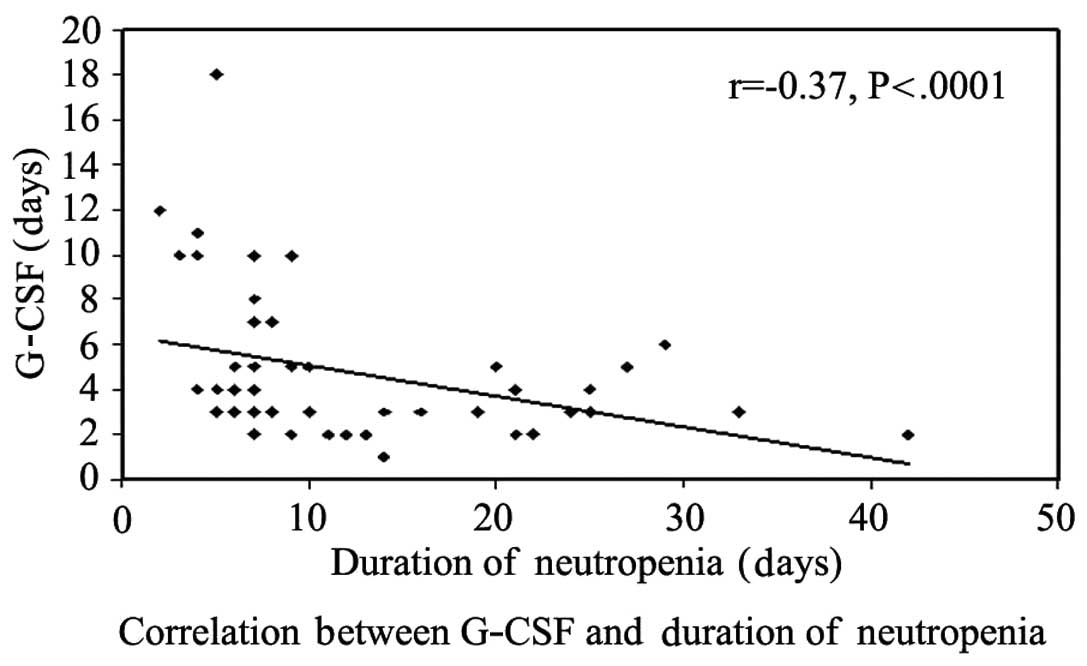
neutropenia duration and G-CSF was obvious in our study, with
faster bone marrow recovery with G-CSF implementation (Fig. 3). Different chemotherapy regimens were
associated with a variable suppressive effect on the bone marrow.
The effect of different protocols on neutrophil dynamics is
presented in Table II.
 | Table II.Effect of treatment protocols on
neutrophil dynamics. |
Table II.
Effect of treatment protocols on
neutrophil dynamics.
| Protocols | Onset of
neutropenia (mean ± SD) | ANC (mean ±
SD) | Duration of
neutropenia (mean ± SD) |
|---|
| Chemotherapy blocks
for neuroblastoma |
|
|
|
|
HDP/VP |
8.8±2.9b |
120.3±138.7a |
6.5±2.58d |
|
CAV |
5.3±4.0b |
226.0±73.0 |
12.3±5.0 |
|
IF/VP |
11±0.0 |
234.0±0.0 |
7.0±0.0d |
| Chemotherapy blocks
for rhabdomyosarcoma |
|
|
|
|
I2Vad2 |
8.8±1.708b |
90.0±.14.1a |
6.5±0.7d |
|
I2Va |
7.5±0.7b |
139.5±142.1a |
7.5±0.7d |
|
VAC |
9.0±0.7 |
474.0±0.0 |
13.0±0.0 |
|
ICE |
9.0±0.0 |
474.0±0.0 |
9.0±0.0 |
| Chemotherapy blocks
for Burkitt's lymphoma |
|
|
|
| COPADM
(methotrexate: 8 gm/m2) |
8.9±2.1 |
183.3±69.5 |
8.1±1.8 |
|
CYVE |
11.7±2.1 |
154.3±106.8a |
5.8±1.3d |
| COPADM
(methotrexate: 3 gm/m2) |
7.0±2.1b |
72.2±19.6a |
5.5±2.4d |
|
CYM |
5.5±007b |
175±35.355 |
5.0±0.0d |
| CCG protocol for
ALL |
|
|
|
|
Induction (standard risk) |
3.7±1.505b |
301.167±107.949 |
15.0±5.831 |
|
Induction (high risk) |
7.6±2.97b |
268.1±117.9 |
15.8±9.1 |
|
Consolidation (standard
risk) |
9.5±3.5 |
147.0±60.8a |
13.5±16.2 |
|
Consolidation (high
risk-standard arm) |
13.3±6.5 |
418.0
±0.0 |
13.7
±11.9 |
|
Consolidation (high
risk-augmented arm) |
13.2±7.7 |
221.6±96.8 |
14.2±8.6 |
| Delayed
intensification-2 (standard risk) |
8.0±0.0b |
127.5±135.0a |
10.5±2.1 |
| Delayed
intensification-1 (standard risk) |
11.4±2.9 |
196.0±72.6 |
13.2±6.1 |
| Delayed
intensification-2 (high risk) |
18.5±0.7 |
234.0±0.0 |
9.5±2.1 |
| Delayed
intensification-1 (high risk) |
12.3±8.7 |
313.7±113.7 |
6.3±3.2d |
|
Maintenance phase |
46.7±26.6c |
310.9±139.3 |
8.6±3.8 |
| Interim
maintenance (standard risk) |
14.7±9.2 |
275.3±153 |
12.8±6.6 |
| Interim
maintenance (high risk) |
19.5±24.7 |
250.5±120 |
10.5±4.9 |
| BFM 2004 protocol
for AML |
|
|
|
| AIE
induction blocks |
5.4±2.1b |
284.6±170 |
23.0±13.6e |
| HAE
block |
8.0±0.0b |
100.0±0.0a |
11.0±0.0 |
| BFM 2002 protocol
for ALL relapse |
|
|
|
| F1
block |
7.5±0.7b |
183.5±17.7a |
24.5±6.4e |
| R1
block |
9.0±0.0 |
468.0±0.0 |
8.5±2.1 |
| R2
block |
13.0±0.0 |
160.0.±0.0a |
5.0±0.0e |
| FLAG
conditioning protocol for BMT |
12.0±0.0 |
54.0±0.0a |
8.0±0.0 |
| ABVD
protocol for Hodgkin lymphoma |
7.75±3.6b |
217.5±33.0 |
18.5±7.7 |
CIN sequelae
Children diagnosed with CIN may experience
infectious and dose-modifying consequences. FN was the leading
complication in 73.5% of our cases, persisting for a mean of
5.7±3.7 days (range, 1–18 days); mucositis, respiratory,
gastrointestinal and skin infections were also documented. The
incidence of infection-related mortality [severe septicemia,
disseminated intravascular coagulation (DIC)] was 6% (3/50) in our
study. Prolonged neutropenia, necessitating chemotherapy dose
reduction, delay or even discontinuation, was also reported. The
complications of CIN are summarized in Fig. 4.
Association of CIN complications with
neutrophil count and duration of neutropenia
An analysis of the association of ANC and duration
of neutropenia with different complications emphasized the
following: First, neutropenic cases complicated with infections,
particularly mucositis and gastrointestinal tract infections,
exhibited a significantly lower ANC and a longer duration of
neutropenia compared with non-infectious cases; and second,
patients who received dose modification exhibited significantly
more prolonged neutropenia compared with those who received
full-dose treatment. However, lower ANC and longer duration of
neutropenia were observed among patients requiring treatment delay
or discontinuation when compared with those who received treatment
on time, but the difference did not reach statistical significance
(Table III).
 | Table III.Association between complications of
CIN and neutrophil count. |
Table III.
Association between complications of
CIN and neutrophil count.
|
| Neutrophil
count | Duration |
|---|
|
|
|
|
|---|
| Complications | Mean (SD) | t (P-value) | Mean (SD) | t (P-value) |
|---|
| Mucositis |
| 2.46 (0.01) |
| 1.79 (0.07) |
|
Absent | 262.3 (123) |
| 9.8 (4.6) |
|
|
Present | 201.2 (131) |
| 12.3 (8.8) |
|
| Respiratory
infection |
| 1.72 (0.06) |
| 0.00 (0.99) |
|
Absent | 249.9 (119) |
| 11.2 (7.6) |
|
|
Present | 205.3 (142) |
| 11.1 (6.9) |
|
| GIT infection |
| 2.26 (0.02) |
| 1.13 (0.26) |
|
Absent | 251.5 (127) |
| 10.6 (5.9) |
|
|
Present | 193.3 (130) |
| 12.2 (9) |
|
| Skin infection |
| 0.32 (0.74) |
| 2.16 (0.03) |
|
Absent | 232 (123) |
| 10.4 (5.8) |
|
|
Present | 222.1 (155) |
| 13.8 (10.4) |
|
| Othersa |
| 1.17 (0.24) |
| 0.78 (0.43) |
|
Absent | 225.8 (129) |
| 11 (7.2) |
|
|
Present | 286 (145) |
| 13.1 (8.7) |
|
| TTT
discontinuation |
| 1.34 (0.18) |
| 1.05 (0.29) |
| No | 223.4 (129) |
| 11.4 (7.5) |
|
|
Yes | 275.5 (136) |
| 9.3 (4.9) |
|
| TTT delay |
| 0.82 (0.4) |
| 1.70 (0.09) |
| No | 225.9 (133) |
| 10.7 (7) |
|
|
Yes | 258.1 (111) |
| 14.1 (8.4) |
|
| Dose reduction |
| 1.24 (0.21) |
| 2.35 (0.02) |
| No | 225.9 (127) |
| 10.8 (6.4) |
|
|
Yes | 294.3 (179) |
| 17.8 (15.8) |
|
Effect of CIN on total treatment
cost
In our study, the mean medical cost of each
neutropenic episode was 9,386.5±6,688.9 Egyptian pounds, divided as
1,574.4±783 for hospital stay, 2,381.7±2,535 for antimicrobials,
3,536±2,123 for supportive treatment, 1,417.3±1,131 for
investigations and 475.8±115.5 for surgical measures. There was a
significant positive correlation between the total cost and the
duration of neutropenia (r=0.66, P<0.001) but not between the
cost and ANC (r=0.1, P>0.05).
Discussion
Therapeutic strategies for cancer continue to
evolve, and chemotherapy regimens continue to play important roles
in cancer treatment (18). Despite
the importance of CIN as a primary dose-limiting toxicity of
chemotherapy, its risks and consequences, particularly among
pediatric patients, have not been fully elucidated. In the present
study, we classified the risk parameters for CIN as patient-,
disease- and regimen-specific. No significant association between
any of the patient characteristics (age, gender and anthropometric
measurements) and the risk of CIN was identified. These findings
are opposite to those reported by several investigators in adult
populations, who documented advanced age and female gender as
significant risk factors (3,14). The hormonal effect of gender on
immunity may be more apparent in older age, as the aging process,
either per se (physiological aging) (19), or due to the associated comorbidities,
such as diabetes, renal disease and hypertension, may exert a
negative effect on neutrophil dynamics (20), thus increasing the risk, incidence,
severity and duration of neutropenia in advanced age (18). A total of 62.8% of neutropenic
episodes in the present study occurred in ALL, 6.2% in acute
myeloid leukemia (AML), 14.1% in lymphomas, and the remaining 16.8%
were associated with solid tumors; these percentages are either
consistent with the previous conclusion that hematological
malignancies are associated with a higher incidence of CIN compared
with solid tumors, due to the underlying disease as well as the
intensity of the required treatment (3,21), or they
merely represent the fact that CIN is a common complication of the
most prevalent childhood malignancy (ALL) (22). However, ANC was not found to be
associated with cancer stage or patient risk grade.
Different chemotherapy protocols were associated
with a variable suppressive effect on the bone marrow; a similar
observation was documented by Lyman et al, who described
certain regimens as ‘more myelotoxic’ compared with others
(23). Induction blocks for the
treatment of ALL and AML were associated with early-onset
neutropenia (3.66±1.5 vs. 5.4±2.1 days, respectively) compared with
the late neutropenia onset in patients who received maintenance
therapy for ALL. In addition, the longest neutropenia episode
occurred in patients treated with induction block for relapsed ALL
and AML. These findings were in concordance with previous studies
reporting that the greatest risk for severe and prolonged
neutropenia was observed during the early treatment cycles
(23–26). These results allowed some clinicians
to consider early cycle hematological response to chemotherapy as a
functional assessment of the effect of treatment on bone marrow
and, subsequently, predict which patients are candidates for
further dose modification or conditioned G-CSF prophylaxis
(27). The high incidence of
neutropenia with early cycles may be explained by the heavier doses
of chemotherapy during induction, while the lower incidence with
subsequent cycles is likely due to dose modifications and
hematopoietic cell adaptation that may occur later on.
Therapy with G-CSF was associated with faster bone
marrow recovery, with a significant negative correlation between
G-CSF and duration of neutropenia (r-0.37, P≤0.001). Likewise,
Ghalaut et al reported a significant shortening of CIN and
FN, as well as of the mean duration of hospitalization (28). These advantages have been documented
by other researchers (3,29,30). In
agreement with our results, a recent study on urological cancer
patients reported a good outcome when G-CSF was administered
(18).
The majority of our patients (73.5%) developed FN,
with a significant positive correlation between the duration of
neutropenia and that of fever (r=0.37, P<0.001). Similarly, FN
was the most commonly recorded complication (61.4%) of systemic
chemotherapy for hematological oncology adult patients in a recent
study conducted in Uruguay (8). By
contrast, Mahmud et al reported a significantly lower
incidence (25–40%) of FN in their series (31). Our high incidence of FN may be
explained by the higher prevalence of underlying hematological
malignancies that increase the risk for FN, as 10–50% of patients
with solid tumors may develop FN, compared with 80% of those with
hematological malignancies (6).
The most common infections encountered in our
neutropenia episodes were mucositis (54.9%), respiratory (45.1%),
gastrointestinal tract (38.9%) and skin (23.9%) infections.
Respiratory tract and skin infections were the most common
according to Boada Burutaran et al (8), while Anunnatsiri et al reported
urinary tract infection, soft tissue infection and bacteremia as
the most common occurrences (32).
Variable sites of infections may be associated with different
invasive procedures that provide a portal of entry for pathogens
(18). Keefe et al reported a
significantly lower incidence of mucositis (10%) among their cases
(33), with a higher incidence of
mucositis in neutropenic children, possibly due to their higher
mitotic index in the oral mucosa compared with adults, with a
higher risk of mucositis with chemotherapeutics.
Neutropenia cases complicated with mucositis and
gastrointestinal tract infections exhibited lower ANC compared with
non-complicated cases, while cases presenting with skin infections
had a significantly longer duration of neutropenia. These findings
had been described in a historical review (34), and were confirmed in a recent study
(8), where the extent and duration of
neutropenia were significantly associated with the risk of
infection.
Neutropenia was responsible for treatment
discontinuation (13.3%), dose delay (13.3%) and dose reduction
(5.3%) in our patients. The incidence of treatment modification in
our study was significantly lower compared with that reported by
Repetto (35) and Ozer (36). We consider delivered dose intensity to
be a major determinant of the outcome (37); thus, every effort was made, including
supportive measures and adjunctive G-CSF, prior to dose
modification or delay.
A total of 6% of our patients succumbed to severe
septicemia and DIC, with similar or even higher rates reported by
previous studies (21,38). This finding highlights the true risk
of devastating infections in these populations, if not aggressively
and promptly managed (4,5).
CIN and related complications have an economic
impact on health care providers (8).
The mean cost for each neutropenia episode in our service was
9,386.5±6,688.9 Egyptian pounds, which was significantly lower
compared with those reported in different economic analyses
(39–41). Government financial support of
chemotherapy and exclusion of indirect non-medical costs from our
calculation, such as lost working hours, may be the causes of our
lower estimated costs.
Although this study is a preliminary survey with a
relatively limited patient sample, our findings are relevant to the
clinical care of pediatric cancer patients in our region. Special
attention to CIN prevention should be directed to hematological
malignancy cases, particularly during the early cycles of
treatment. Severe and prolonged neutropenia is life-threatening and
requires aggressive management.
References
|
1
|
te Poele EM, Tissing WJ, Kamps WA and de
Bont ES: Risk assessment in fever and neutropenia in children with
cancer: What did we learn? Crit Rev Oncol Hematol. 72:45–55. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozer H, Armitage JO, Bennett CL, Crawford
J, Demetri GD, Pizzo PA, Schiffer CA, Smith TJ, Somlo G, Wade JC,
et al: 2000 update of recommendations for the use of hematopoietic
colony-stimulating factors: Evidence-based, clinical practice
guidelines. American Society of Clinical Oncology Growth Factors
Expert Panel. J Clin Oncol. 18:3558–3585. 2000.PubMed/NCBI
|
|
3
|
Crawford J, Dale DC and Lyman GH:
Chemotherapy-induced neutropenia: Risks, consequences and new
direction for its management. Cancer. 100:228–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weycker D, Barron R, Kartashov A, Legg J
and Lyman GH: Incidence, treatment and consequences of
chemotherapy-induced febrile neutropenia in the inpatient and
outpatient settings. J Oncol Pharm Pract. 20:190–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynn JJ, Chen KF, Weng YM and Chiu TF:
Risk factors associated with complications in patients with
chemotherapy-induced febrile neutropenia in emergency department.
Hematol Oncol. 31:189–196. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dulisse B, Li X, Gayle JA, Barron RL,
Ernst FR, Rothman KJ, Legg JC and Kaye JA: A retrospective study of
the clinical and economic burden during hospitalizations among
cancer patients with febrile neutropenia. J Med Econ. 16:720–735.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh
MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA and Wingard
JR: Clinical practice guideline for the use of antimicrobial agents
in neutropenic patients with cancer: 2010 update by the Infectious
Diseases Society of America. Clin Infect Dis. 52:e56–e93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boada Burutaran M, Guadagna1 R, Grille S,
Stevenazzi M, Guillermo C and Diaz L: Results of high-risk
neutropenia therapy of hematology-oncology patients in a university
hospital in Uruguay. Rev Bras Hematol Hemoter. 37:28–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Villela L and Bolaños-Meade J: Acute
myeloid leukaemia: Optimal management and recent developments.
Drugs. 71:1537–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper KL, Madan J, Whyte S, Stevenson MD
and Akehurst RL: Granulocyte colony-stimulating factors for febrile
neutropenia prophylaxis following chemotherapy: Systematic review
and meta-analysis. BMC Cancer. 11:4042011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J,
Cross SJ, et al: 2006 update of recommendations for the use of
white blood cell growth factors: An evidence-based clinical
practice guideline. J Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aapro MS, Cameron DA, Pettengell R,
Bohlius J, Crawford J, Ellis M, Kearney N, Lyman GH, Tjan-Heijnen
VC, Walewski J, et al: EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphomas and solid tumors. Eur J Cancer. 42:2433–2453. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lyman GH, Kuderer N, Greene J and Balducci
L: The economics of febrile neutropenia: Implications for the use
of colony-stimulating factors. Eur J Cancer. 34:1857–1864. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hosmer W, Malin J and Wong M: Development
and validation of a prediction model for the risk of developing
febrile neutropenia in the first cycle of chemotherapy among
elderly patients with breast, lung, colorectal and prostate cancer.
Support Care Cancer. 19:333–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita M, Tokunaga S, Ikegame S, Harada E,
Matsumoto T, Uchino J, Watanabe K and Nakanishi Y: Identifying risk
factors for refractory febrile neutropenia in patients with lung
cancer. J Infect Chemother. 18:53–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodey GP and Rolston KV: Management of
fever in neutropenic patients. J Infect Chemother. 7:1–9. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hughes WT, Armstrong D, Bodey GP, Bow EJ,
Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL and
Young LS: 2002 guidelines for the use of antimicrobial agents in
neutropenic patients with cancer. Clin Infect Dis. 34:730–751.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yasufuku T, Shigemura T, Tanaka K, Arakawa
S, Miyake H and Fujisawa M: Risk factors for refractory febrile
neutropenia in urological chemotherapy. J Infect Chemo. 19:211–216.
2013. View Article : Google Scholar
|
|
19
|
Balducci L and Extermann M: Management of
cancer in the older person: A practical approach. Oncologist.
5:224–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aslani A, Smith RC, Allen BJ, Pavlakis N
and Levi JA: The predictive value of body protein for
chemotherapy-induced toxicity. Cancer. 88:796–803. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christopher R and Friese RN: Chemotherapy
induced neutropenia: Important new data to guide nursing assessment
and management. Adv Stud Nurs. 4:21–25. 2006.
|
|
22
|
Buffler PA, Kwan ML, Reynods P and Urayama
KY: Environmental and genetic risk factors for childhood leukemia:
Appraising the evidence. Cancer Invest. 23:60–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lyman GH, Kuderer NM and Balducci L:
Cost-benefit analysis of granulocyte colony-stimulating factor in
the management of elderly cancer patients. Curr Opin Hematol.
9:207–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gomez H, Hidalgo M, Casanova L, Colomer R,
Pen DL, Otero J, Rodríguez W, Carracedo C, Cortés-Funes H and
Vallejos C: Risk factors for treatment-related death in elderly
patients with aggressive non-Hodgkin's lymphoma: Results of a
multivariate analysis. J Clin Oncol. 16:2065–2069. 1998.PubMed/NCBI
|
|
25
|
Caggiano V, Stolshek BS, Delgado DJ and
Carter WB: First and all cycle febrile neutropenia hospitalizations
(FNH) and costs in intermediate grade non-Hodgkin's lymphoma (IGL)
patients on standard-dose CHOP therapy. Blood. 98:431a(abstract
1810). 2001.
|
|
26
|
Meza L, Baselga J, Holmes FA, Liang B and
Breddy J: Incidence of febrile neutropenia (FN) is directly related
to duration of severe neutropenia (DSN) after myelosuppressive
chemotherapy. Proc Am Soc Clin Oncol. 21:255b(abstract 2840).
2002.
|
|
27
|
Wilson-Royalty M, Lawless G, Palmer C and
Brown R: Predictors for chemotherapy-related severe or febrile
neutropenia: A review of the clinical literature. J Oncol Pharm
Pract. 7:141–147. 2002. View Article : Google Scholar
|
|
28
|
Ghalaut PS, Sen R and Dixit G: Role of
granulocyte colony stimulating factor (G-CSF) in chemotherapy
induced neutropenia. J Assoc physician India. 56:942–944. 2008.
|
|
29
|
Heil G, Hoelzer D, Sanz MA, Lechner K, Liu
Yin JA, Papa G, Noens L, Szer J, Ganser A, O'Brien C, et al: A
randomized, double blind, placebo-controlled phase III study of
filgrastim in remission induction and consideration therapy for
adults with de novo acute myeloid leukemia. The International Acute
Myeloid Leukemia Study Group. Blood. 90:4710–4718. 1997.PubMed/NCBI
|
|
30
|
Larson RA, Dodge RK, Linker CA, Stone RM,
Powell BL, Lee EJ, Schulman P, Davey FR, Frankel SR, Bloomfield CD,
et al: A randomized controlled trial of filgrastim during remission
induction and consolidation chemotherapy for adults with acute
lymphoblastic leukemia. CALGB study 9111. Blood. 92:1556–1564.
1998.PubMed/NCBI
|
|
31
|
Mahmud S, Ghafoor T and Badsha S:
Bacterial infections in pediatric patients with chemotherapy
induced neutropenia. JPMA. 54:2372004.
|
|
32
|
Anunnatsiri S, Chansung K, Chetchotisakd P
and Sirijerachai C: Febrile neutropenia: A retrospective study in
Srinagarind Hospital. J infect Dis Antimicrob agents. 15:115–122.
1998.
|
|
33
|
Keefe DM, Schubert MM, Elting LS, Sonis
ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB,
Hutchins RD and Peterson DE: Updated clinical practice guidelines
for the prevention and treatment of mucositis. Cancer. 109:820–831.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bodey GP, Buckley M, Sathe YS and
Freireich EJ: Quantitative relationships between circulating
leukocytes and infection in patients with acute leukemia. Ann
Intern Med. 64:328–340. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Repetto L: Incidence and clinical impact
of chemotherapy induced myelotoxicity in cancer patients: An
observational retrospective survey. Crit Rev Oncol Hematol.
72:170–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ozer H: The timing of chemotherapy-induced
neutropenia and its clinical and economic impact. Oncology
(Williston Park). 20:11–15. 2006.PubMed/NCBI
|
|
37
|
Citron ML, Berry DA, Cirrincione CT, Hudis
C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R,
Ingle JN, et al: Randomized trial of dose-dense versus
conventionally scheduled and sequential versus concurrent
combination chemotherapy as postoperative adjuvant treatment of
node-positive primary breast cancer: First report of Intergroup
Trial C9741/cancer and Leukemia Group B Trial 9741. J Clin Oncol.
21:1431–1439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Caggiano V, Weiss R, Rickert TS and
Linde-Zwirble WT: Incidence, cost and mortality of neutropenia
hospitalization associated with chemotherapy. Cancer.
103:1916–1924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuderer N, Cosler LE, Crawford J, Dale DC
and Lyman GH: Cost and mortality associated with febrile
neutropenia in adult cancer patients. Proc Am Soc Clin Oncol.
21:250a(abstract 998). 2002.
|
|
40
|
Gandhi SK, Arguelles L and Boyer JG:
Economic impact of neutropenia and febrile neutropenia in breast
cancer: Estimates from two national databases. Pharmacotherapy.
21:684–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weycker D, Malin J, Edelsberg J, Glass A,
Gokhale M and Oster G: Cost of neutropenic complications of
chemotherapy. Ann Oncol. 19:454–460. 2008. View Article : Google Scholar : PubMed/NCBI
|