Introduction
Testicular germ cell tumors (TGCT) represent the
most common solid tumor type affecting young men aged between 18
and 35-years-old (1). The incidence
and mortality are known to vary across countries (2). Despite the increase in its incidence
during the last decade, particularly in Western countries, the
number of patients dying from the disease is stable due to
increased cure rates (3). Improved
survival stems predominantly from increased staging accuracy,
adequate early treatment based on a multidisciplinary approach, the
use of platinum-based regimens, careful follow-up and salvage
therapies. In the oncologic center of excellence, cure rates as
high as 90% at 10 years were achieved (4). These good results draw attention to the
quality of life of survivors. In addition, the importance of this
issue becomes more apparent when considering that the vast majority
of these survivors are <40-years-old (1).
Notably, to achieve these high rates of cure, even
in high risk patients, several treatment strategies and regimens
are often delivered. These modalities are associated with a
significant quantity of complications and a negative impact on
quality of life (5). Sperm
abnormalities are frequently observed in patients with TGCT
(6). Furthermore, orchiectomy,
chemotherapy and radiation therapy can also impair fertility
(7). The risk for solid secondary
tumor types increases with a younger age at radiotherapy or
chemotherapy, and remains significantly elevated for at least 35
years (8). The risk of leukemia is
also associated with the dose of chemotherapy, with a cumulative
dose-disease association regarding cisplatin and acute myeloid
leukemia (9). Pulmonary toxicity and
infections are common problems in the long-term setting (10). Hypogonadism and metabolic syndrome are
prevalent among testicular cancer survivors (11). Therefore, subsequent cardiovascular
events are higher compared with the general population (12). Psychological disturbances, emotional
liabilities, physical and/or cognitive tiredness, and depression
are common long-term complaints (13). Long-term close follow-up due to the
possibility of recurrence expose such patients to anxiety and
uncertainty (13). Understandably,
all these events adversely affect the quality of life, which is
deteriorated in >1/3 of patients (14).
The present study analyzed the data of a cohort of
patients with TGCT treated at the Jules Bordet Institute. The aim
of the present study was to report the experiences of TGCT
management over the last 10 years, particularly in terms of
survival for each prognostic group of TGCT. The long-term
follow-up, the impact of treatment on patient metabolic parameters
and the impact of the treatment on the quality of life were
investigated.
Materials and methods
The present study involved a cohort of 115
consecutive patients with GCT, treated and followed-up at the Jules
Bordet Institute, University ULB of Brussels (Brussels, Belgium)
between September 2000 and December 2010. The collected data were
pooled and retrospectively analyzed. The project was approved by
the Institute Jules Bordet Ethics Review Committee in August 2013.
All patients with testicular germ cell neoplastic disease, having
received their initial treatment and under periodic follow-up care
at the Jules Bordet Institute, were included. Patients with
histology other than germ cell neoplastic disease were
excluded.
Socio-demographic characteristics, physical
examination and tumor markers, including human chorionic
gonadotropin (HCG), α fetoprotein (AFP) and lactate dehydrogenase
(LDH), were obtained for all patients. A complete blood test
analysis was also performed at diagnosis. Local disease was
assessed by a testicular echography, whereas retroperitoneal
extension and distant metastases were assessed using a
thoracoabdominal computed tomography scan. A bone scan was
requested in symptomatic patients or in men with elevated alkaline
phosphatase. A magnetic resonance imaging scan of the brain was
reserved for poor risk metastatic patients or patients with
neurological symptoms. The modalities of treatment administered to
patients followed the principles of the European Society of Medical
Oncology (ESMO) guidelines (15,16). For
stage I seminoma, surveillance was the preferred strategy. In the
presence of certain risk factors, including rete testis
infiltration or tumor size >4 cm, one course of carboplatin was
applied or radiotherapy (20 Gy in 10 fractions to para aortic
target volume). In stage IIA seminoma, with the presence of lymph
nodes sized 1–2 cm, both cisplatin-based chemotherapy and radiation
therapy to para aortic and ipsilateral iliac lymph nodes are valid
options. In stage II B/C seminoma, three cycles of bleomycin,
etoposide and cisplatin (BEP) was the standard of care. In the case
of contra-indication to the use of bleomycin, four cycles of
etoposide-cisplatin (EP) were alternatively used. In stage III
seminoma, three cycles of bleomycin, etoposide and cisplatin (BEP)
was the standard of care for patients with good prognosis,
according to the International Germ Cell Cancer Collaborative Group
(IGCCCG), and four cycles of BEP for those with an intermediate
prognosis. In the case of residual tumor post-chemotherapy, a
2-fluor-2-deoxy-D-glucose positron emission tomography (PET) scan
was performed if the lesion was >3 cm. In the case of a positive
PET, surgical resection of the residual lesion was performed.
Patients with non-seminoma (NS)GCT stage I were divided into two
categories based on the presence or absence of vascular invasion.
In the absence of lymphovascular invasion, surveillance was
preferred, whereas high risk patients received two cycles of BEP.
Primary chemotherapy for stage II and III NSGCT consisted of three
or four courses of BEP, depending on the IGCCCG risk
classification. Patients with good prognosis received three cycles
of BEP, or four cycles of EP if bleomycin was contra-indicated.
Patients with intermediate risk were treated with four cycles of
BEP or four cycles of etoposide, ifosfamide and cisplatin (VIP) if
bleomycin raised concerns for lung toxicity. Prophylactic
administration of growth factors, including granulocyte colony
stimulating factor (GCSF), was recommended only in cases of
infectious complications following the first courses of
chemotherapy. In the case of relapse, the regimen most frequently
used was VIP, followed by paclitaxel, ifosfamide and cisplatin
(TIP). High-dose chemotherapy in association with autologous stem
cell transplantation was used in selected patients.
The post-treatment follow-up respected the
principles of the ESMO guidelines. Statistical analysis was
performed using the SAS System version 9.4 (SAS Institute Inc.,
Cary, NC, USA). Descriptive summary statistics were used to
describe the patient's population and the administered treatments.
Treatment-associated toxicities were reported using proportions.
Survival analysis was performed using non-parametric analysis
(Kaplan-Meier estimates) and the log-rank test was used to compare
Kaplan-Meier curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline and tumor characteristics of the study
population were summarized in Tables
I and II. Overall, a total of
115 patients (median age, 31-years-old) were enrolled in the
present study, with a median follow-up of 6 years. Cryptorchidism
was the most common risk factor for TGCTs identified in 8% of the
enrolled patients. Of the patients, 4 had co-morbidities. Positive
family history for GCT was observed in 1% of patients and 10% of
patients had a family history of another cancer type. At the moment
of diagnosis, 44 (38%) patients were single and only 19 (17%) had
children. The life habits of the patients were also documented at
diagnosis. More than half of the patients failed to answer the
question concerning the use of marijuana, and of the 56 patients
who did answer, 6 (5%) admitted to be a regular user. With regards
to the localization of the primary tumor, the testis was the most
common place with an equal distribution among the two testes. With
the exception of 5 patients, the remaining patients were initially
treated with surgery. The pathology of the specimen was described
in Table I. In stage I, 11 patients
were followed-up without treatment, whereas 35 and 11 patients
received adjuvant chemotherapy and radiation therapy, respectively.
The patients (47.5%) were classified as stage II and III and
received chemotherapy based on the ESMO guidelines. In terms of
prognosis, 70% of patients were classified with a good, 13% as
intermediate and 17% as poor diagnosis, according to the IGCCCG
classification (17). Pulmonary
function tests, renal clearance of creatinine and Eastern
Cooperative Oncology Group performance status at the initiation of
chemotherapy, as well as the extent of the disease were summarized
in Table III. The modalities of the
first regimen and treatment-associated toxicities were summarized
in Table IV. The incidence of
febrile neutropenia was 11%. No allergic reaction was observed and
in only 3% of patients, bleomycin was either avoided or
discontinued due to lung toxicity. A total of 17% of patients
reported interpersonal and professional concerns during
treatment.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| A, Risk factors |
|---|
|
|---|
| Characteristic | No. patients
(n=115) | % |
|---|
| Cryptorchidism |
|
|
| No | 88 | 76 |
| Yes | 9 | 8 |
|
Unknown | 18 | 16 |
| Orchidopexy |
|
|
| No | 92 | 80 |
| Yes | 8 | 7 |
|
Unknown | 15 | 13 |
| Trauma |
|
|
| No | 95 | 83 |
| Yes | 4 | 3 |
|
Unknown | 16 | 14 |
| Atrophy |
|
|
| No | 95 | 83 |
| Yes | 5 | 4 |
|
Unknown | 15 | 13 |
| Gonadal
dysgenesis |
|
|
| No | 99 | 86 |
| Yes | 1 | 1 |
|
Unknown | 15 | 13 |
| Hypogonadism |
|
|
| No | 97 | 84 |
| Yes | 3 | 3 |
|
Unknown | 15 | 13 |
| Genetic
syndrome |
|
|
| No | 99 | 86 |
|
Yes | 0 | 0 |
|
Unknown | 16 | 14 |
|
| B, Past medical
history |
|
| Characteristic | No. patients
(n=115) | % |
|
| Liver
dysfunction |
|
|
| No | 17 | 15 |
|
Yes | 1 | 1 |
|
Unknown | 97 | 84 |
| Sub fertility |
|
|
| No | 16 | 14 |
|
Yes | 2 | 2 |
|
Unknown | 97 | 84 |
| Sterility |
|
|
| No | 17 | 15 |
|
Yes | 1 | 1 |
|
Unknown | 97 | 84 |
| Other |
|
|
|
Yes | 10 | 9 |
|
Unknown/no | 105 | 91 |
|
| C, Further
socio-demographic characteristics |
|
| Characteristic | No. patients
(n=115) | % |
|
| Family history of
germ cell cancer |
|
|
| No | 94 | 82 |
|
Yes | 1 | 1 |
|
Unknown | 20 | 17 |
| Family history of
another cancer type |
|
|
| No | 81 | 70 |
|
Yes | 12 | 10 |
|
Unknown | 22 | 20 |
| Marital status |
|
|
|
Married | 23 | 20 |
|
Partner | 9 | 8 |
|
Single | 44 | 38 |
|
Unknown | 39 | 34 |
| Children at
diagnosis |
|
|
| No | 55 | 48 |
|
Yes | 19 | 17 |
|
Unknown | 41 | 35 |
| Allergy |
|
|
| No | 75 | 65 |
|
Yes | 9 | 8 |
|
Unknown | 31 | 27 |
|
| D, Life habits |
|
| Characteristic | No. patients
(n=115) | % |
|
| Sport |
|
|
| No | 31 | 27 |
|
Yes | 20 | 17 |
|
Unknown | 64 | 56 |
| Tobacco |
|
|
| No | 37 | 32 |
|
Yes | 46 | 40 |
|
Unknown | 32 | 28 |
| Alcohol |
|
|
| No | 66 | 57 |
|
Yes | 17 | 15 |
|
Unknown | 32 | 28 |
| Drugs |
|
|
| No | 57 | 50 |
|
Yes | 2 | 1 |
|
Unknown | 56 | 49 |
| Marijuana |
|
|
| No | 50 | 44 |
|
Yes | 6 | 5 |
|
Unknown | 59 | 51 |
 | Table II.Tumor characteristics. |
Table II.
Tumor characteristics.
| Characteristic | No. patients
(n=115) | % |
|---|
| Primary
localization |
|
|
|
Testis | 101 | 88 |
|
Mediastinum |
1 | 1 |
|
Retroperitoneum | 13 | 11 |
| Initial
histology |
|
|
|
Seminoma | 67 | 58 |
|
Embryonal carcinoma | 19 | 17 |
|
Choriocarcinoma |
2 | 2 |
|
Teratoma |
2 | 2 |
| Yolk
sac tumor |
1 | 1 |
|
Mixed | 17 | 15 |
|
Other |
7 | 6 |
| Prognostic group
ICCCG |
|
|
|
Good | 81 | 70 |
|
Intermediate | 15 | 13 |
|
Poor | 19 | 17 |
| Curative
treatment |
|
|
|
Surgery, RT, chemo |
4 | 3 |
| Sugery,
RT, no chemo | 31 | 27 |
|
Surgery, no RT, chemo | 66 | 57 |
| Sugery,
no RT, no chemo |
9 | 8 |
| No
surgery, RT, chemo |
3 | 3 |
| No
surgery, no RT, chemo |
2 | 2 |
| Initial tumor
markers |
|
|
| AFP
(ng/ml) |
|
|
|
No. patients
used | 68 | – |
|
Mean ± standard
deviation | 247±778 | – |
|
Median
(min-max) | 3.8 (0.1 to
4956) | – |
| hCG
(mU/ml) |
|
|
|
No. patients
used | 61 | – |
|
Mean ± standard
deviation | 465±1411 | – |
|
Median
(min-max) | 1.3 (0.1 to
6705) | – |
| LDH
(UI/l) |
|
|
|
No. patients
used | 55 | – |
|
Mean ± standard
deviation | 771±1283 | – |
|
Median
(min-max) | 480 (8–9390) | – |
| CEA
(µg/l) |
|
|
|
No. patients
used | 33 | – |
|
Mean ± standard
deviation | 2.3±1.6 | – |
|
Median
(min-max) | 2.5 (0.1–7.7) | – |
 | Table III.ECOG performance status, extend of
the disease, pulmonary function testing, dilution of carbon
monoxide and cryopreservation of semen, in 81 patients at
initiation of chemotherapy. |
Table III.
ECOG performance status, extend of
the disease, pulmonary function testing, dilution of carbon
monoxide and cryopreservation of semen, in 81 patients at
initiation of chemotherapy.
| Characteristic | No. patients | % |
|---|
| ECOG performance
status |
|
|
| 0 | 66 | 89 |
| 1 | 7 | 10 |
| 2 | 0 | 0 |
| 3 | 1 | 1 |
| Extend of the
disease |
|
|
| Lymph
node | 36 | 42 |
|
Lung | 9 | 11 |
|
Liver | 2 | 2 |
|
Bone | 1 | 1 |
|
CNS | 6 | 7 |
| No
meta | 31 | 36 |
|
Unknown | 1 | 1 |
| Measurable
lesions |
|
|
| No | 9 | 17 |
|
Yes | 43 | 83 |
| Stage |
|
|
| IA | 33 | 45 |
| IB | 4 | 5 |
|
IIA | 2 | 3 |
|
IIB | 3 | 4 |
|
IIC | 1 | 1 |
|
IIIA | 8 | 11 |
|
IIIB | 9 | 12 |
|
IIIC | 14 | 19 |
| RFE |
|
|
|
Normal | 56 | 69 |
|
Abnormal | 4 | 5 |
| Missing
info | 21 | 26 |
|
Cryopreservation |
|
|
| No | 7 | 9 |
|
Yes | 49 | 61 |
|
Unknown | 25 | 30 |
| Renal clearance
EDTA |
|
|
|
Normal | 57 | 70 |
|
Abnormal | 2 | 3 |
| Missing
info | 22 | 27 |
 | Table IV.Modalities of first line
chemotherapy. |
Table IV.
Modalities of first line
chemotherapy.
| Characteristic | No. patients | % |
|---|
| Type of
chemotherapy |
|
|
|
BEP | 37 | 51 |
| EP | 2 | 3 |
|
VIP | 3 | 4 |
|
TIP | – | – |
|
Carboplatin | 24 | 33 |
|
Other | 6 | 9 |
| High dose
chemotherapy |
|
|
| No | 70 | 97 |
|
Yes | 2 | 3 |
| Stem cell
transfusion |
|
|
| No | 70 | 97 |
|
Yes | 2 | 3 |
| Number of cycles
administered |
|
|
| No.
patients used | 70 |
|
| Mean ±
standard deviation | 2.6±1.3 |
|
| Median
(min-max) | 3 (1- 6) |
|
| Platine cumulative
dose |
|
|
| No.
patients used | 41 |
|
| Mean ±
standard deviation | 317±83 |
|
| Median
(min-max) | 300 (100–500) |
|
| Bleomycin
cumulative dose |
|
|
| No.
patients used | 37 |
|
| Mean ±
standard deviation | 256±80 |
|
| Median
(min-max) | 270 (90–450) |
|
| Concomitant
medications |
|
|
| No | 10 | 16 |
|
Primary | 52 | 81 |
|
Secondary | 2 | 3 |
At the long-term follow-up, 8 patients had succumbed
to mortality; 6 had succumbed to their disease and 2 to unrelated
causes. Relapses were observed in 11 patients and 2 patients
developed a second primary malignancy. The regimen most frequently
used for relapses was VIP. Among survivors, complete and partial
remission of the disease was achieved in 89 and 5% of patients,
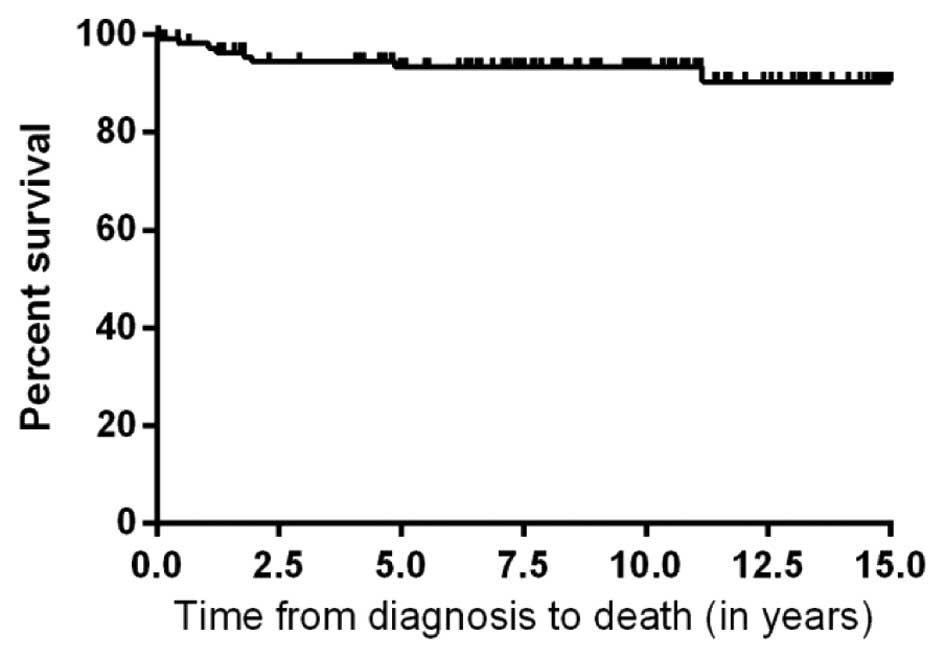
respectively. The 5-year overall survival rate was 93% (Fig. 1). The event free survival rate at 5
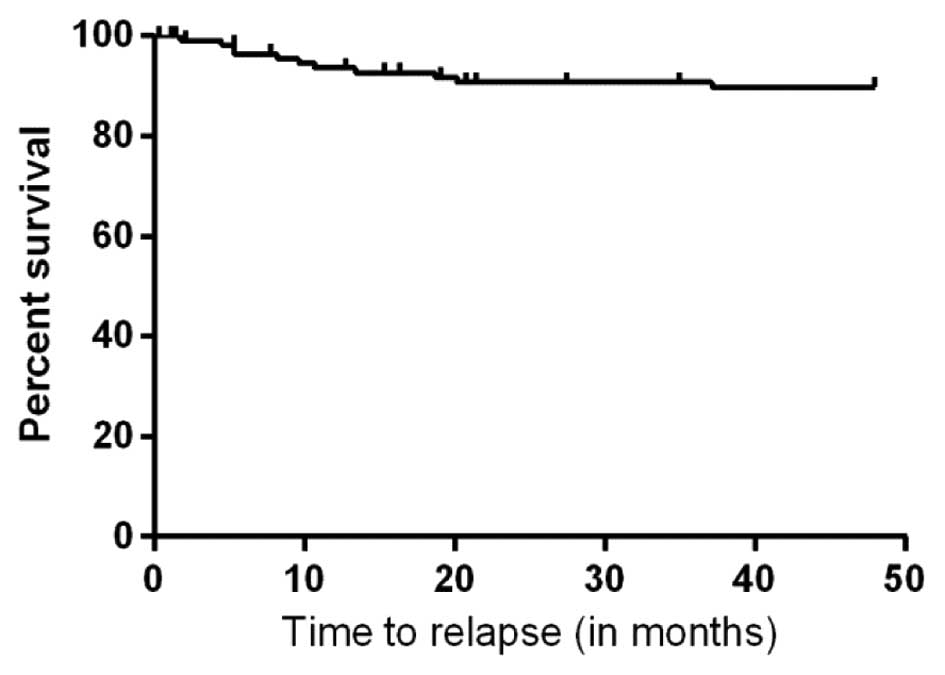
years was 90% (Fig. 2). The poor
prognosis group had a statistically significant shorter duration to
relapse compared with the groups of good or intermediate prognosis
(P=0.002; Fig. 3).
Of the patients, 1/3 managed to have children
following treatment. The most often used modality was in
vitro fertilization. During the follow-up period, 8% of
patients divorced and 8% changed profession. Cannabis withdrawal
was noted in 2 patients and 60% began or continued physical
activity. Weight, blood pressure and pulse were not statistically
different between the first year post-treatment and the final
follow-up. Of these patients, 1/5 st a new medication to control
treatment-associated side effects, including hypercholesterolemia,
hypogonadism and depression.
Discussion
The present study highlighted the good oncological
outcomes of patients suffering with testicular germ cell cancer.
Furthermore, high compliance rates were observed for starting and
maintaining physical activity, as well as avoiding weight gain.
However, cannabis withdrawal was less likely among survivors as
only 2/6 regular users stopped consumption. Only 1/5 of patients
started a new medication to control blood pressure, dyslipidemia,
hypogonadism or depression. The latter symptom was less frequent in
patients living in couples. Of note, only 1/4 patients had fathered
children prior to the diagnosis and 1/3 managed to father children
following the treatment. The vast majority of patients were healthy
males with no co-morbidities, and cryptorchidism was the most
common risk factor identified in the cohort of patients. Notably,
only 1% of patients had a positive family history of TGCT. These
findings are in accordance with a previous meta-analysis indicating
that cryptorchidism and familial history of testicular cancer
augmented the risk of TGCT to 4.8 and 3, respectively (18). An estimated 1.4% of men with newly
diagnosed TGCT reported a positive family history for this cancer
(18). A pooled segregation analysis
supported the existence of a Mendelian inheritance in multiple-case
TGCT families, with the evidence markedly favoring an autosomal
recessive mechanism. Candidate gene analyses have had mixed
results. Germline mutations in three Xq27 genes, KIT or DND1, as
well as germline variants in the PDE11A have been associated with
the risk of familial TGCT. More recent analyses have implicated
KITLG, SPRY4, and BAK1 as sporadic testicular cancer susceptibility
loci (19). Currently, medical
literature does not support an association between alcohol
consumption and testicular cancer, however, one previous study
offered evidence of an association between smoking and moderate
risk of TGCT (20,21). Based on a previous epidemiological
analysis, marital status was an independent predictor factor of
improved overall survival and cancer-specific survival (22).
Chemotherapy regimens were well-tolerated and only
11% of patients developed febrile neutropenia. The use of GCSF
concomitantly to the chemotherapy for testicular cancer is
debatable. Bokemeyer et al (23) demonstrated that the concomitant use of
GCSF with chemotherapy ameliorated chemotherapy-induced
myelosuppression, allowed increased dose intensity of the treatment
and generated more peripheral blood stem cells (23). In their study, the risk of neutropenic
infections was <20% for the group of good risk patients with
metastatic disease, and prophylactic use of GCSF was not routinely
recommended. For intermediate and poor risk patients where
aggressive cis-platinum based regimens were used, the risk of
febrile neutropenia was between 20 and 70%, and prophylactic use of
GCSF was beneficial. At present, the concomitant use of GCSF with
high dose chemotherapy is advisable. In the present study, no
statistically significant benefit in terms of overall survival was
noted between patients taking GCSF with chemotherapy compared with
patients without GCSF. However, more prospective studies are
required in order to define the exact role of GCSF in the treatment
of TGCTs.
In terms of survival, the present results are in the
line with those of contemporary series (24). A multi-institutional European study
performed in 27 countries revealed the 10-year overall survival
rates of 96% for testicular cancer (4). In the USA, the 5-year overall survival
is 99% for localized disease, 96% for regional disease and 74% for
advanced disease, based on the data of the National Cancer
Institute's Surveillance, Epidemiology and Results (25). Prognostic factors associated with
improved survival rates commonly found in the literature are:
Younger age, marital status and early stage, all reach ~100% of 1
year overall survival for stage I disease (26). In general, the constant improvement of
overall cure rates is attributed substantially to early diagnosis
and the use of cis-platinum-based chemotherapy regimens.
In terms of quality of life, several previous
studies suggested that treatment of TGCT has a great impact on the
overall status of the patients. From the physical point of view,
the cardiovascular system is most commonly affected. A study by
Huddart et al (12) showed
that cardiovascular-associated co-morbidities are increased by 100%
following treatment for testicular cancer (12). Bokemeyer et al (23) reported that in addition to Raynaud's
phenomenon, high blood pressure, higher serum cholesterol,
ototoxicity and peripheral neuropathy occurred (23). These late complications may affect the
quality of life of TGCT survivors and were observed also in the
present population. Previous studies revealed anxiety to be present
in a great number of TGCT survivors causing fatigue, and decreased
physical and mental ability (27).
Social functioning was also impaired, suggesting the impact of the
treatment on their ability to integrate and interact with others.
Interpersonal problems and divorce were signaled in 17% of the
current patients (28).
Fertility studies in men treated for testicular
cancer have predominantly focused on follicle stimulating hormone
levels and sperm quality parameters (29). Chemotherapy has the greatest impact on
fertility, followed by radiotherapy and retroperitoneal lymph node
dissection (30,31). Fosså et al (28) reported that the paternity rate in
1,814 testicular cancer survivors was 71% and the duration from
diagnosis until the birth of the first child after diagnosis was
6.6 years (28). The low paternity
rate found in the present study is probably a hazardous finding
associated with the small number of patients and missing
information on paternity for many of them.
TGCT survivors have a risk of developing second
malignancies ~65–90% higher compared with age-matched controls. The
relative risk for leukemia, associated with the previous use of
etoposide, ranges between 3.5 and 4.5 and appears usually within
ten years following the completion of treatment (32). In the present study, the incidence of
a second primary solid cancer and treatment-associated acute
leukemia is in accordance with the incidence reported in the
literature.
In conclusion, the present findings are consistent
with the results of most European and international studies in
terms of epidemiological features and survival for patients with
TGCT. Long-term surveillance and psychological support of patients
with TGCT are mandatory. Lifestyle modifications are mandatory in
order to avoid metabolic syndrome and cardiovascular morbidity. The
usage of chemotherapy and radiation therapy in the adjuvant setting
must be restricted to only the high risk patients. Prospective
studies focusing on the quality of life of survivors are required
in order to improve the management of testicular germ cell cancer
survivors.
References
|
1
|
Sui W, Morrow DC, Bermejo CE and
Hellenthal NJ: Trends in testicular cancer survival: A large
population-based analysis. Urology. 85:1394–1398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Richiardi L, Ekbom A, Pukkala E,
Cuninkova M and Møller H: Trends in testicular cancer incidence and
mortality in 22 European countries: Continuing increases in
incidence and declines in mortality. Int J Cancer. 118:3099–3111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
La Vecchia C, Bosetti C, Lucchini F,
Bertuccio P, Negri E, Boyle P and Levi F: Cancer mortality in
Europe, 2000–2004 and an overview of trends since 1995. Ann Oncol.
21:1323–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trama A, Mallone S, Nicolai N, Necchi A,
Schaapveld M, Gietema J, Znaor A, Ardanaz E and Berrino F: RARECARE
Working Group: Burden of testicular, paratesticular and
extragonadal germ cell tumours in Europe. Eur J Cancer. 48:159–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sender L and Zabokrtsky KB: Adolescent and
young adult patients with cancer: A milieu of unique features. Nat
Rev Clin Oncol. 12:465–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petersen PM, Skakkebaek NE and Giwercman
A: Gonadal function in men with testicular cancer: Biological and
clinical aspects. APMIS. 106:24–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomlinson M, Meadows J, Kohut T, Haoula Z,
Naeem A, Pooley K and Deb S: Review and follow-up of patients using
a regional sperm cryopreservation service: Ensuring that resources
are targeted to those patients most in need. Andrology. 3:709–716.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng AK, Kenney LB, Gilbert ES and Travis
LB: Secondary malignancies across the age spectrum. Semin Radiat
Oncol. 20:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howard R, Gilbert E, Lynch CF, Hall P,
Storm H, Holowaty E, Pukkala E, Langmark F, Kaijser M, Andersson M,
et al: Risk of leukemia among survivors of testicular cancer: A
population-based study of 42,722 patients. Ann Epidemiol.
18:416–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis LB, Beard C, Allan JM, Dahl AA,
Feldman DR, Oldenburg J, Daugaard G, Kelly JL, Dolan ME, Hannigan
R, et al: Testicular cancer survivorship: Research strategies and
recommendations. J Natl Cancer Inst. 102:1114–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lackner JE, Märk I, Schatzl G, Marberger M
and Kratzik C: Hypogonadism and androgen deficiency symptoms in
testicular cancer survivors. Urology. 69:754–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huddart RA, Norman A, Shahidi M, Horwich
A, Coward D, Nicholls J and Dearnaley DP: Cardiovascular disease as
a long-term complication of treatment for testicular cancer. J Clin
Oncol. 21:1513–1523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malec JF, Romsaas EP, Messing EM, Cummings
KC and Trump DL: Psychological and mood disturbance associated with
the diagnosis and treatment of testis cancer and other
malignancies. J Clin Psychol. 46:551–557. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fleer J, Hoekstra HJ, Sleijfer DT and
Hoekstra-Weebers JE: Quality of life of survivors of testicular
germ cell cancer: A review of the literature. Support Care Cancer.
12:476–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huddart RA and Kataja VV: ESMO Guidelines
Task Force: ESMO minimum clinical recommendations for diagnosis,
treatment and follow-up of testicular seminoma. Ann Oncol. 16(Suppl
1): i40–i42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huddart RA and Purkalne G: ESMO Guidelines
Task Force: ESMO minimum clinical recommendations for diagnosis,
treatment and follow-up of mixed or non-seminomatous germ cell
tumors (NSGCT). Ann Oncol. 16(Suppl 1): i37–i39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
International germ cell consensus
classification: A prognostic factor based staging system for
metastatic germ-cell cancers. International Germ Cell Cancer
Collaborative Group. J Clin Oncol. 15:594–603. 1997.PubMed/NCBI
|
|
18
|
Dieckmann KP and Pilchlmeier U: Clinical
epidemiology of testicular germ cell tumors. World J Urol. 22:2–14.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greene MH, Kratz CP, Mai PL, Mueller C,
Peters JA, Bratslavsky G, Ling A, Choyke PM, Premkumar A, Bracci J,
et al: Familial testicular germ cell tumors in adults: 2010 summary
of genetic risk factors and clinical phenotype. Endocr Relat
Cancer. 17:R109–R121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava A and Kreiger N: Cigarette
smoking and testicular cancer. Cancer Epidemiol Biomarkers Prev.
13:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shinn EH, Swartz RJ, Thornton BB, Spiess
PE, Pisters LL and Basen-Engquist KM: Testis cancer survivors'
health behaviors: Comparison with age-matched relative and
demographically matched population controls. J Clin Oncol.
28:2274–2279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abern MR, Dude AM and Coogan CL: Marital
status independently predicts testis cancer survival-an analysis of
the SEER database. Urol Oncol. 30:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bokemever C, kuczyk MA, Köhne H, Einsele
H, Kynast B and Schmoll HJ: Hematopoietic growth factors and
treatment of testicular cancer: Biological interactions, routine
use and dose-intensive chemotherapy. Ann Hematol. 72:1–9. 1996.
View Article : Google Scholar
|
|
24
|
Damjanov I and Wewer-Albrechtsen N:
Testicular germ cell tumors and related research from a historical
point of view. Int J Dev Biol. 57:197–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v1.2, Cancer Incidence and
Mortality Worldwide: IARC CancerBase No. 10 (Internet).
International Agency for Research on Cancer (Lyon, France).
2010.http://globocan.iarc.frAccessed. December
12–2014PubMed/NCBI
|
|
26
|
Shanmugalingam T, Soultati A, Chowdhury S,
Rudman S and Van Hemelrijck M: Global incidence and outcome of
testicular cancer. Clin Epidemiol. 5:417–427. 2013.PubMed/NCBI
|
|
27
|
Reilley MJ, Jacobs LA, Vaughn DJ and
Palmer SC: Health behaviors among testicular cancer survivors. J
Community Support Oncol. 12:121–128. 2014.PubMed/NCBI
|
|
28
|
Fosså SD, Dahl AA and Loge JH: Fatigue,
anxiety and depression in long-term survivors of testicular cancer.
J Clin Oncol. 21:1249–1254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hotaling JM, Patel DP, Vendryes C,
Lopushnyan NA, Presson AP, Zhang C, Muller CH and Walsh TJ:
Predictors of sperm recovery after cryopreservation in testicular
cancer. Asian J Androl. 18:35–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Magelssen H, Brydøy M and Fosså SD: The
effects of cancer and cancer treatments on male reproductive
function. Nat Clin Pract Urol. 3:312–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brydøy M, Fosså SD, Klepp O, Bremnes RM,
Wist EA, Wentzel-Larsen T and Dahl O: Paternity following treatment
for testicular cancer. Nat Cancer Inst. 97:1580–1588. 2005.
View Article : Google Scholar
|
|
32
|
Richiardi L, Scélo G, Boffetta P, Hemminki
K, Pukkala E, Olsen JH, Weiderpass E, Tracey E, Brewster DH,
McBride ML, et al: Second malignancies among survivors of germ-cell
testicular cancer: A pooled analysis between 13 cancer registries.
Int J Cancer. 120:623–631. 2007. View Article : Google Scholar : PubMed/NCBI
|