Introduction
Colorectal cancer (CRC) is the third and second most
commonly diagnosed cancer in men and women, respectively, with an
estimated 1.4 million new cases and nearly 700,000 deaths in 2012
worldwide (1). Approximately 60% of
CRC patients are aged >70 years (2). The number of elderly individuals is
increasing worldwide due to the increase in the average life span.
As a result, the number of middle-old (75–84 years) and oldest-old
patients (>85 years) diagnosed with CRC is expected to increase.
Although potentially curative resection is not possible in
approximately one-fourth of the patients (3), surgery is the most reliable treatment
modality. In the preoperative management of elderly patients,
cardiovascular risk, respiratory function and the presence of
comorbidities are thoroughly assessed. However, nutritional status
assessment may be overlooked in routine preoperative evaluation,
despite the fact that undernutrition is associated with an
increased risk of poor tissue healing and impaired immune function
(4), and previous studies reporting
that poor nutritional status was associated with worse survival of
elderly patients who underwent surgery for lung, ovarian and
primary peritoneal cancer (4,5).
Body mass index (BMI) is a useful tool in clinical
practice for assessing adult nutritional status, and individuals
are considered to be underweight if their BMI is <18.5
kg/m2. A BMI<18.5 kg/m2 also indicates
undernutrition (6). Several studies
conducted among subjects of a broad age range reported that
underweight status was a significant predictor of poorer overall
survival in CRC (7–13). However, to the best of our knowledge,
no study has yet been conducted to investigate the association
between underweight status and survival outcome in elderly CRC
patients.
The aim of this study was to investigate whether
underweight status was associated with a worse survival outcome in
elderly patients undergoing curative surgery for CRC.
Materials and methods
Patient population
A total of 113 Japanese patients with pathologically
confirmed stage I–III CRC, aged ≥75 years, who underwent curative
surgery between January, 2004 and June, 2012 at the Department of
Surgery, Omori Red Cross Hospital (Tokyo, Japan) were
retrospectively analyzed. The patients were followed up for ≥3
years or until death. Patients with cancer of other organs were
excluded. The medical charts of the 113 patients were retrieved
from our registry, and clinical, pathological and survival data
were collected. The factors included in this study were age,
gender, Charlson Comorbidity Index (14), the calculation of which did not
include cancer diagnoses, American Society of Anesthesiologists
physical status, tumor site, disease stage according to the Union
for International Cancer Control (15), histology and BMI at the time of
diagnosis. BMI was calculated as weight in kilograms divided by the
square of the height in meters (kg/m2). Patients were
divided into underweight (BMI<18.5 kg/m2) and
non-underweight (BMI≥18.5 kg/m2) groups, according to
the World Health Organization (WHO) classification (6). The associations between 3-year overall
survival (OS) or cancer-specific survival (CSS) and the patients'
clinicopathological data were analyzed. The 3-year OS, CSS and
recurrence-free survival (RFS) curves were then estimated for each
group using the Kaplan-Meier method. The review board of the
hospital approved the study protocol.
Statistical analysis
The associations between BMI and the
clinicopathological parameters were assessed using the Pearson's
Chi-square or Fisher's exact tests, as appropriate. Cox regression
analyses were performed to analyze the survival outcome in
univariate and multivariate analyses. Variables that were
significant in the univariate analysis were examined in the
multivariate analysis. OS, CSS and RFS were determined from the
date of surgery to the date of death from any cause,
cancer-specific death and recurrence, respectively. The
Kaplan-Meier survival curves were compared using the log-rank test.
All reported P-values were two-tailed and those <0.05 were
considered to indicate statistically significant differences. All
data were analyzed using EZR version 1.24 software for Windows
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria) (16).
Results
Patient characteristics
Of the 113 patients, 20 (17.6%) died within 3 years
of surgery: 14 patients succumbed to CRC, whereas the remaining 6
patients died from other causes. The median BMI was 21.3
kg/m2 (range, 12.6–30.2 kg/m2), and 1 patient
(0.8%) had a BMI of ≥30 kg/m2. A total of 24 patients
(21%) were in the underweight and 89 (79%) in the non-underweight
group.
The clinical and pathological characteristics of the
patients, stratified by BMI, are listed in Table I. The two groups were well-balanced
regarding all factors evaluated, although underweight patients
exhibited a trend toward a higher Charlson Comorbidity Index score
compared with patients who were not underweight (P=0.060).
 | Table I.Baseline characteristics of the CRC
patients stratified by BMI. |
Table I.
Baseline characteristics of the CRC
patients stratified by BMI.
|
|
Non-underweighta |
Underweightb |
|
|---|
|
|
|
|
|
|---|
| Characteristics | n (%) | n (%) | P-value |
|---|
| Age (years) |
|
| 0.25 |
|
<85 | 73 (82.0) | 17 (70.8) |
|
| ≥85 | 16 (18.0) | 7
(29.2) |
|
| Gender |
|
| 0.25 |
|
Female | 47 (52.8) | 16 (66.7) |
|
| Male | 42 (47.2) | 8
(33.3) |
|
| Charlson comorbidity
index |
|
| 0.060 |
| 0, 1 | 78 (87.6) | 17 (70.8) |
|
| ≥2 | 11 (12.4) | 7
(29.2) |
|
| ASA physical
status |
|
| 0.19 |
| I,
II | 63 (70.8) | 13 (54.2) |
|
| III,
IV | 26 (29.2) | 11 (45.8) |
|
| Tumor site |
|
| 0.10 |
|
Colon | 69 (77.5) | 14 (58.3) |
|
|
Rectum | 20 (22.5) | 10 (41.7) |
|
| Stage |
|
| 0.45 |
| I,
II | 66 (74.2) | 16 (66.7) |
|
| III | 23 (25.8) | 8
(33.3) |
|
| Histology |
|
| 0.67 |
|
Differentiated | 83 (93.3) | 22 (91.7) |
|
|
Undifferentiated | 6 (6.7) | 2 (8.3) |
|
Analysis of OS and CSS
The results of the univariate and multivariate Cox
regression analyses for 3-year OS are shown in Table II. In the univariate analysis, the
factors significantly associated with poorer 3-year OS were
advanced disease stage (P=0.001) and underweight status (P=0.023).
The multivariate Cox regression analysis demonstrated that advanced
disease stage [hazard ratio (HR)=4.31; 95% confidence interval
(CI): 1.76–10.58; P=0.001] and underweight status (HR=2.65; 95% CI:
1.08–6.50; P=0.033) were independently associated with worse 3-year
OS.
 | Table II.Cox regression analyses of 3-year
overall survival. |
Table II.
Cox regression analyses of 3-year
overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
| ≥85 vs.
<85 | 1.76 | 0.67–4.58 | 0.24 |
|
| Gender |
|
| Male
vs. female | 1.60 | 0.66–3.86 | 0.29 |
|
| Charlson
comorbidity index |
|
| ≥2 vs.
0, 1 | 1.36 | 0.45–4.07 | 0.57 |
|
| ASA physical
status |
|
| III, IV
vs. I, II | 1.72 | 0.71–4.16 | 0.22 |
|
| Tumor site |
|
| Rectum
vs. colon | 0.86 | 0.31–2.39 | 0.78 |
|
| Stage |
|
| III vs.
I, II | 4.47 | 1.82–10.95 | 0.001 | 4.31 | 1.76–10.58 | 0.001 |
| Histology |
|
|
Undifferentiated vs.
differentiated | 3.16 | 0.92–10.80 | 0.066 |
|
| BMI |
|
|
Underweighta vs. non-underweightb | 2.82 | 1.15–6.91 | 0.023 | 2.65 | 1.08–6.50 | 0.033 |
The results of the univariate and multivariate Cox
regression analyses for 3-year CSS are shown in Table III. The factors significantly
associated with a poorer 3-year CSS were advanced disease stage
(P<0.001), undifferentiated histology (P=0.016) and underweight
status (P=0.034). The multivariate Cox regression analysis revealed
that advanced disease stage (HR=6.69; 95% CI: 2.08–21.44; P=0.001),
undifferentiated histology (HR=4.37; 95% CI: 1.15–16.62; P=0.030),
and underweight status (HR=3.51; 95% CI: 1.16–10.60; P=0.025) were
independently associated with a worse 3-year CSS.
 | Table III.Cox regression analyses of 3-year
cancer-specific survival. |
Table III.
Cox regression analyses of 3-year
cancer-specific survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
| ≥85 vs.
<85 | 1.11 | 0.30–3.98 | 0.87 |
|
| Gender |
|
| Male
vs. female | 1.73 | 0.60–4.99 | 0.30 |
|
| Charlson
comorbidity index |
|
| ≥2 vs.
0, 1 | 0.91 | 0.20–4.07 | 0.90 |
|
| ASA physical
status |
|
| III, IV
vs. I, II | 1.58 | 0.55–4.57 | 0.39 |
|
| Tumor site |
|
| Rectum
vs. colon | 1.04 | 0.32–3.33 | 0.93 |
|
| Stage |
|
| III vs.
I, II | 7.32 | 2.29–23.40 | <0.001 | 6.69 | 2.08–21.44 | 0.001 |
| Histology |
|
|
Undifferentiated vs.
differentiated | 4.75 | 1.32–17.07 | 0.016 | 4.37 | 1.15–16.62 | 0.030 |
| BMI |
|
|
Underweighta vs. non-underweightb | 3.13 | 1.08–9.02 | 0.034 | 3.51 | 1.16–10.60 | 0.025 |
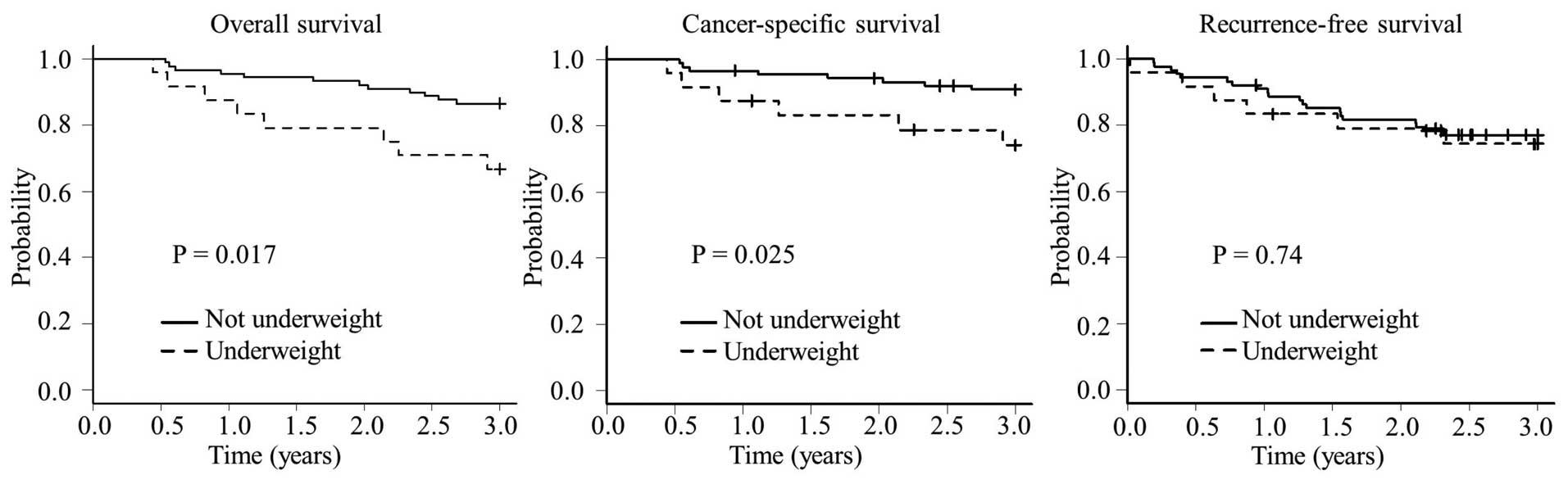
Patients in the underweight group had a
significantly worse 3-year OS rate compared with those in the
non-underweight group (66.7 vs. 86.5%, respectively; P=0.017) and
CSS rate (74.1 vs. 90.9%, respectively; P=0.025) (Fig. 1). There was no significant difference
in the 3-year RFS rate between the underweight and non-underweight
groups (74.3 vs. 77.1%; P=0.74).
Discussion
The association between underweight status and the
prognosis of CRC patients has been reported in several studies
conducted among patients of all ages, and underweight patients have
been shown to have a worse OS (7–13).
However, Hines et al reported that underweight patients were
on average older compared with those in other BMI categories, and
more frequently had moderate or severe comorbidities compared with
their younger counterparts (10);
thus, older age and comorbidities were possible confounding factors
when attempting to determine the association between BMI categories
and survival outcome in CRC patients (10). In this study, among elderly CRC
patients, we found that underweight status, but not the Charlson
Comorbidities Index score, was an independent risk factor for
all-cause and cancer-specific mortality. Therefore, the worse
prognosis of underweight patients was not solely attributed to
their older age and more severe comorbidities.
It remains unclear whether underweight status exerts
a significant negative effect on CSS or RFS in patients with CRC
(7–13). Doria et al reported an
increased risk of cancer-related death among underweight patients
with colon cancer. Two other studies reported that underweight
status per se was not a significant risk factor for CSS in
CRC, and that underweight patients tended to succumb to non-cancer
events, which was attributed, at least in part, to an underlying
comorbid illness (8,12). In regards to RFS, it has previously
been reported that underweight patients have a higher risk of
cancer recurrence (12,13). Conversely, other investigators found
no association between underweight status and RFS (7,11). In the
present study, we observed that underweight patients had a worse
CSS but similar RFS with non-underweight patients. One possible
explanation for these findings is that underweight status may be
associated with more severe comorbidities and a poor performance
status, thus preventing effective chemotherapeutic treatment or
surgery when the disease recurs (17). Indeed, underweight patients in this
study exhibited a trend toward a higher Charlson Comorbidity Index
score and were less likely to receive salvage treatment for
recurrent disease compared with patients who were not underweight
(16 vs. 75%, respectively; data not shown).
Underweight status in elderly patients may be
associated with the loss of muscle and fat mass due to sarcopenia
and/or cachexia. Sarcopenia in elderly individuals was defined as
the ‘progressive loss of muscle mass and strength with a risk of
adverse outcomes, such as disability, poor quality of life and
death’ by the Special Interest Group of the European Sarcopenia
Working Group in 2010 (18), and it
is recognized as a multifactorial geriatric syndrome. The term
‘sarcopenia’ is used specifically to denote the loss of muscle mass
and strength associated with aging, distinct from muscle loss due
to other causes, such as immobility or neurological damage.
Sarcopenia is independently associated with an increased risk of
functional impairment, falls, disability and mortality in the
elderly (19). The prevalence of
sarcopenia is 50% in individuals aged ≥80 years (18), and several reports demonstrated that
sarcopenia is a negative prognostic factor in malignancies,
including melanoma (20),
hepatocellular carcinoma (21) and
diffuse large B-cell lymphoma (22).
Cachexia may be a cause of loss of body fat mass
(23). It has been reported that the
loss of body fat content late in life is associated with premature
death, micronutrient deficiencies, frailty, increased hospital
admission, an increased risk of disability from falls and delayed
recovery from injury (24–27). In addition, loss of body fat content
has been reported to be associated with shorter survival in
advanced cancer patients, although the underlying mechanism of this
association has not yet been fully elucidated (28,29). One
possible explanation is that fat represents the main energy store
of the body, and it may be one of the components predicting
malnutrition-related risks of mortality and morbidity; thus, body
fat confers survival advantages in elderly patients (30).
Although BMI is a simple and useful tool in clinical
practice for assessing adult nutritional status, two recent studies
revealed that it cannot be used to assess individual components of
body weight, such as regional fat distribution or muscle volume;
thus, muscular individuals may be incorrectly categorized as
overweight or obese (31,32). The authors of those studies also
suggested that other parameters denoting body composition, such as
fat mass or muscle mass assessed by bioelectrical impedance
analyses, dual-energy X-ray absorptiometry and magnetic resonance
imaging or computed tomography imaging, would be more useful
predictors of survival compared with BMI (31,32).
Further studies are required to evaluate the association between
BMI and other parameters, and to identify better predictors of
appropriate body composition.
The limitations of the present study include its
retrospective design, small sample size and lack of data such as
physical activity, diet, smoking status and changes in body weight
prior to surgery. In regard to the sample size, only 1 (0.8%) of
the 113 patients had a BMI of ≥30 kg/m2. However, this
reflects the distribution of BMI categories in Japan, where only a
relatively small proportion of the middle-old and oldest-old
populations have a BMI of ≥30 kg/m2 (33).
In conclusion, the results of the present study
indicate that underweight status is an independent poor prognostic
factor in elderly CRC patients, which may exert an effect on
treatment and post-treatment surveillance. Further studies are
required to validate our findings and elucidate the mechanism
underlying the negative effect of underweight status on the
survival of these patients. The development of effective
nutritional interventions may contribute to a better prognosis in
such patients.
Acknowledgements
The authors would like to thank Ms. Yukako Oikawa
for her clerical assistance.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Millan M, Merino S, Caro A, Feliu F,
Escuder J and Francesch T: Treatment of colorectal cancer in the
elderly. World J Gastrointest Oncol. 7:204–220. 2015.PubMed/NCBI
|
|
3
|
Nelson H, Petrelli N, Carlin A, Couture J,
Fleshman J, Guillem J, Miedema B, Ota D and Sargent D: National
Cancer Institute Expert Panel: Guidelines 2000 for colon and rectal
cancer surgery. J Natl Cancer Inst. 93:583–596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiorelli A, Vicidomini G, Mazzella A,
Messina G, Milione R, Di Crescenzo VG and Santini M: The influence
of body mass index and weight loss on outcome of elderly patients
undergoing lung cancer resection. Thorac Cardiovasc Surg.
62:578–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alphs HH, Zahurak ML, Bristow RE and
Díaz-Montes TP: Predictors of surgical outcome and survival among
elderly women diagnosed with ovarian and primary peritoneal cancer.
Gynecol Oncol. 103:1048–1053. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bailey KV and Ferro-Luzzi A: Use of body
mass index of adults in assessing individual and community
nutritional status. Bull World Health Organ. 73:673–680.
1995.PubMed/NCBI
|
|
7
|
Meyerhardt JA, Tepper JE, Niedzwiecki D,
Hollis DR, McCollum AD, Brady D, O'Connell MJ, Mayer RJ, Cummings
B, Willett C, et al: Impact of body mass index on outcomes and
treatment-related toxicity in patients with stage II and III rectal
cancer: Findings from Intergroup Trial 0114. J Clin Oncol.
22:648–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dignam JJ, Polite BN, Yothers G, Raich P,
Colangelo L, O'Connell MJ and Wolmark N: Body mass index and
outcomes in patients who receive adjuvant chemotherapy for colon
cancer. J Natl Cancer Inst. 98:1647–1654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doria-Rose VP, Newcomb PA, Morimoto LM,
Hampton JM and Trentham-Dietz A: Body mass index and the risk of
death following the diagnosis of colorectal cancer in
postmenopausal women (United States). Cancer Causes Control.
17:63–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hines RB, Shanmugam C, Waterbor JW, McGwin
G Jr, Funkhouser E, Coffey CS, Posey J and Manne U: Effect of
comorbidity and body mass index on the survival of African-American
and Caucasian patients with colon cancer. Cancer. 115:5798–5806.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinicrope FA, Foster NR, Sargent DJ,
O'Connell MJ and Rankin C: Obesity is an independent prognostic
variable in colon cancer survivors. Clin Cancer Res. 16:1884–1893.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chin CC, Kuo YH, Yeh CY, Chen JS, Tang R,
Changchien CR, Wang JY and Huang WS: Role of body mass index in
colon cancer patients in Taiwan. World J Gastroenterol.
18:4191–4198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinicrope FA, Foster NR, Yothers G, Benson
A, Seitz JF, Labianca R, Goldberg RM, Degramont A, O'Connell MJ and
Sargent DJ: Adjuvant Colon Cancer Endpoints (ACCENT) Group: Body
mass index at diagnosis and survival among colon cancer patients
enrolled in clinical trials of adjuvant chemotherapy. Cancer.
119:1528–1536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International union against cancer (UICC) TNM classification of
malignant tumors (7th). Wiley-Blackwell. Oxford: 2009.
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel GS, Ullah S, Beeke C, Hakendorf P,
Padbury R, Price TJ and Karapetis CS: Association of BMI with
overall survival in patients with mCRC who received chemotherapy
versus EGFR and VEGF-targeted therapies. Cancer Med. 4:1461–1471.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fielding RA, Vellas B, Evans WJ, Bhasin S,
Morley JE, Newman AB, van Abellan Kan G, Andrieu S, Bauer J,
Breuille D, et al: Sarcopenia: An undiagnosed condition in older
adults. Current consensus definition: Prevalence, etiology and
consequences. International working group on sarcopenia. J Am Med
Dir Assoc. 12:249–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janssen I, Heymsfield SB and Ross R: Low
relative skeletal muscle mass (sarcopenia) in older persons is
associated with functional impairment and physical disability. J Am
Geriatr Soc. 50:889–896. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabel MS, Lee J, Cai S, Englesbe MJ,
Holcombe S and Wang S: Sarcopenia as a prognostic factor among
patients with stage III melanoma. Ann Surg Oncol. 18:3579–3585.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meza-Junco J, Montano-Loza AJ, Baracos VE,
Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR and Sawyer
MB: Sarcopenia as a prognostic index of nutritional status in
concurrent cirrhosis and hepatocellular carcinoma. J Clin
Gastroenterol. 47:861–870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lanic H, Kraut-Tauzia J, Modzelewski R,
Clatot F, Mareschal S, Picquenot JM, Stamatoullas A, Leprêtre S,
Tilly H and Jardin F: Sarcopenia is an independent prognostic
factor in elderly patients with diffuse large B-cell lymphoma
treated with immunochemotherapy. Leuk Lymphoma. 55:817–823. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ali S and Garcia JM: Sarcopenia, cachexia
and aging: Diagnosis, mechanisms and therapeutic options-a
mini-review. Gerontology. 60:294–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delmi M, Rapin CH, Bengoa JM, Delmas PD,
Vasey H and Bonjour JP: Dietary supplementation in elderly patients
with fractured neck of the femur. Lancet. 335:1013–1016. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tayback M, Kumanyika S and Chee E: Body
weight as a risk factor in the elderly. Arch Intern Med.
150:1065–1072. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pamuk ER, Williamson DF, Madans J, Serdula
MK, Kleinman JC and Byers T: Weight loss and mortality in a
national cohort of adults, 1971–1987. Am J Epidemiol. 136:686–697.
1992.PubMed/NCBI
|
|
27
|
Mowé M, Bøhmer T and Kindt E: Reduced
nutritional status in an elderly population (>70 y is probable
before disease and possibly contributes to the development of
disease. Am J Clin Nutr. 59:317–324. 1994.PubMed/NCBI
|
|
28
|
Murphy RA, Wilke MS, Perrine M, Pawlowicz
M, Mourtzakis M, Lieffers JR, Maneshgar M, Bruera E, Clandinin MT,
Baracos VE and Mazurak VC: Loss of adipose tissue and plasma
phospholipids: Relationship to survival in advanced cancer
patients. Clin Nutr. 29:482–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fouladiun M, Körner U, Bosaeus I, Daneryd
P, Hyltander A and Lundholm KG: Body composition and time course
changes in regional distribution of fat and lean tissue in
unselected cancer patients on palliative care-correlations with
food intake, metabolism, exercise capacity, and hormones. Cancer.
103:2189–2198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouillanne O, Dupont-Belmont C, Hay P,
Hamon-Vilcot B, Cynober L and Aussel C: Fat mass protects
hospitalized elderly persons against morbidity and mortality. Am J
Clin Nutr. 90:505–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez MC, Pastore CA, Orlandi SP and
Heymsfield SB: Obesity paradox in cancer: New insights provided by
body composition. Am J Clin Nutr. 99:999–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujiwara N, Nakagawa H, Kudo Y, Tateishi
R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami
T, et al: Sarcopenia, intramuscular fat deposition, and visceral
adiposity independently predict the outcomes of hepatocellular
carcinoma. J Hepatol. 63:131–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
National Surveys on Cardiovascular
Diseases in 2000. http://www.mhlw.go.jp/toukei/list/junkanki_chousa.htmlAccessed.
June 31–2015(In Japanese).
|