Introduction
Inflammatory myofibroblastic tumor (IMT) is a
distinct neoplasm characterized by spindle cell proliferation and
an inflammatory infiltrate (1). IMTs
located in the retroperitoneum are relative rare (2). The management of this type of tumor may
be challenging, as there are currently no established protocols and
the tumors are occasionally unresectable due to their large size
and proximity to vital structures. We herein present a case of a
retroperitoneal IMT metastatic to the rectum, which was effectively
controlled by chemotherapy following unsuccessful surgical
resection and radiofrequency ablation.
Case report
The patient was a 60-year-old male who was admitted
to a local hospital due to upper abdominal pain for 5 months. The
patient described the pain as continuous and dull, radiating to the
left flank, and he reported a weight loss of 12 kg over the past 5
months. The physical examination was unremarkable. A computed
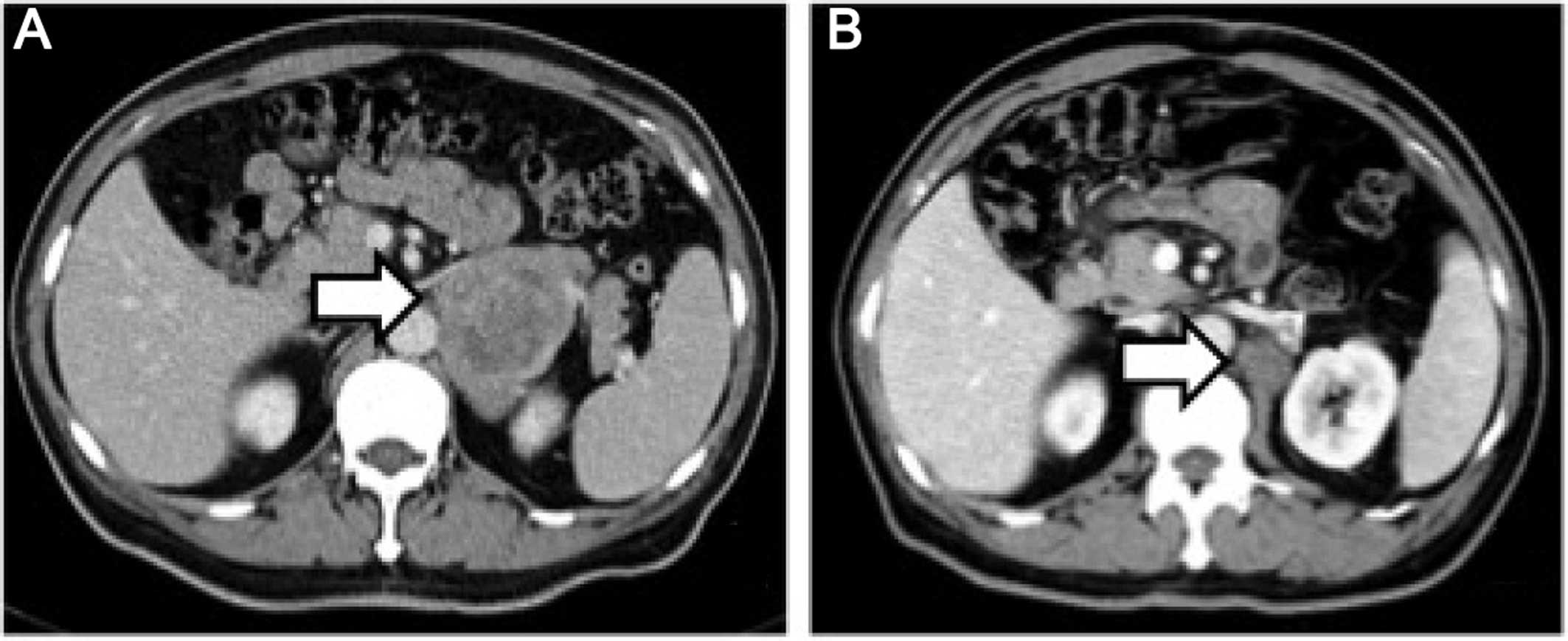
tomography (CT) scan of the abdomen and pelvis revealed a solid
mass in the left adrenal area. The mass measured 6.7×5.1 cm and its
CT value was 30 Hounsfield units. The density of the mass was
enhanced with intravenous contrast administration (Fig. 1A). Non-retroperitoneal lymph nodes
were observed on cross-sectional imaging. A diagnosis of
retroperitoneal tumor was hypothesized, but the presence of an
adrenal mass could not be excluded. The patient was then referred
to our institution and subsequently underwent laparoscopic surgery
for the resection of the retroperitoneal mass and the right adrenal
gland. Macroscopically, the mass was irregular, firm, measuring 9
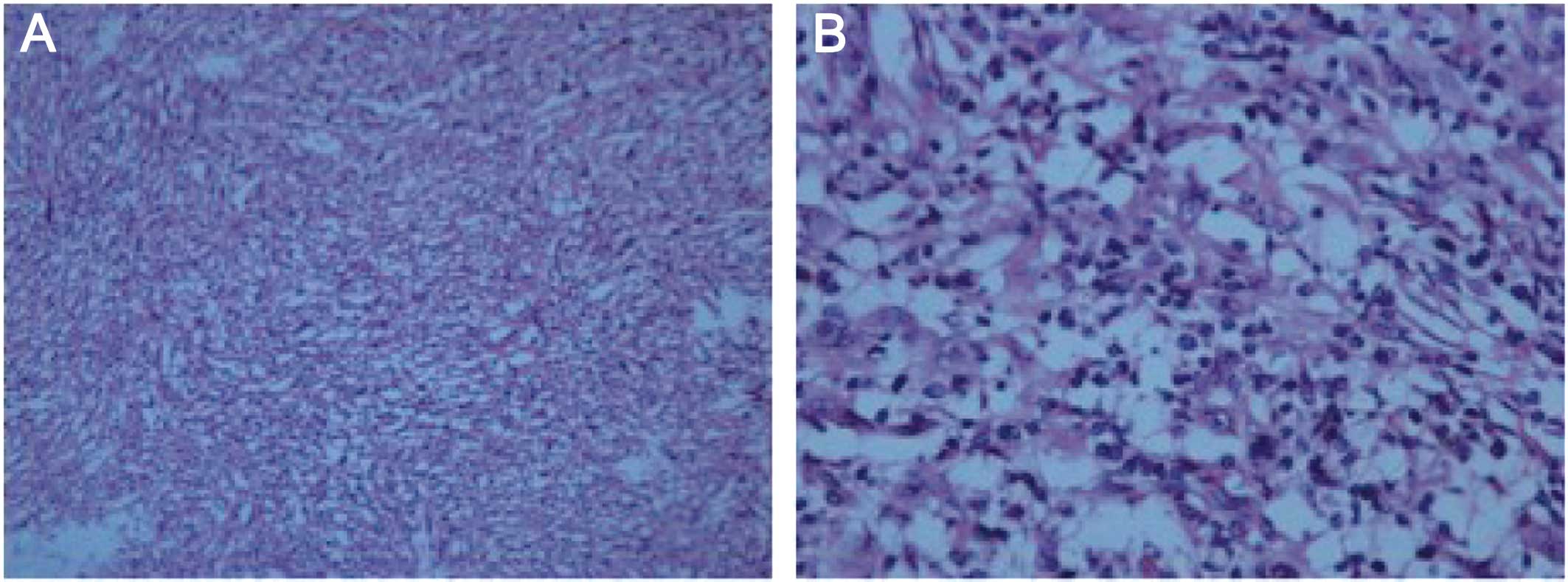
cm in greatest diameter. Histological examination revealed loosely
arranged spindle cells with admixed collagen bundles and scattered
inflammatory cells (Fig. 2A), mainly
comprising lymphocytes and plasma cells (Fig. 2B). The proliferation extended into the
adjacent nerves, fatty tissue and adrenal gland. The surgical
margin was positive for tumor invasion. The immunohistological
examination of the tumor was positive for CD35, CD163, vimentin and
Ki67 (10%), and negative for CD21, CD23, CD34, pancytokeratin,
S-100, desmin, smooth muscle antigen and anaplastic lymphoma kinase
(ALK)-1. A follow-up CT of the abdomen and pelvis revealed
progression of the tumor 2 months after surgical resection. The
tumor was sized 2.6×2.3 cm and was located between the aorta and
the left diaphragmatic angle (Fig.
1B). The patient refused further treatment and no action was
taken, except for close surveillance. Five months after the
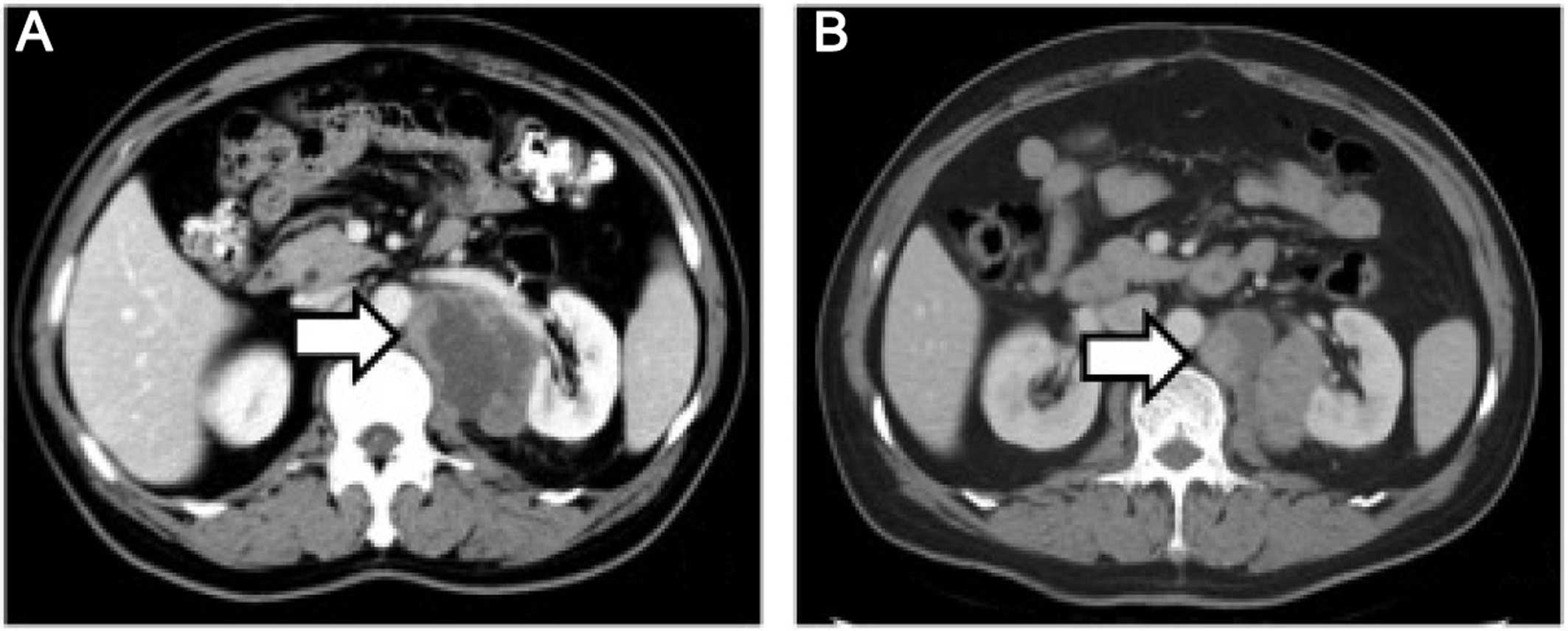
surgery, a repeat CT of the abdomen and pelvis revealed that the
size of the mass had increased to 5.8×4.3×6.5 cm (Fig. 3A). The patient underwent CT-guided
radiofrequency ablation of the retroperitoneal tumor at the Jiangsu
Cancer Hospital; however, shortly after the second surgery, an
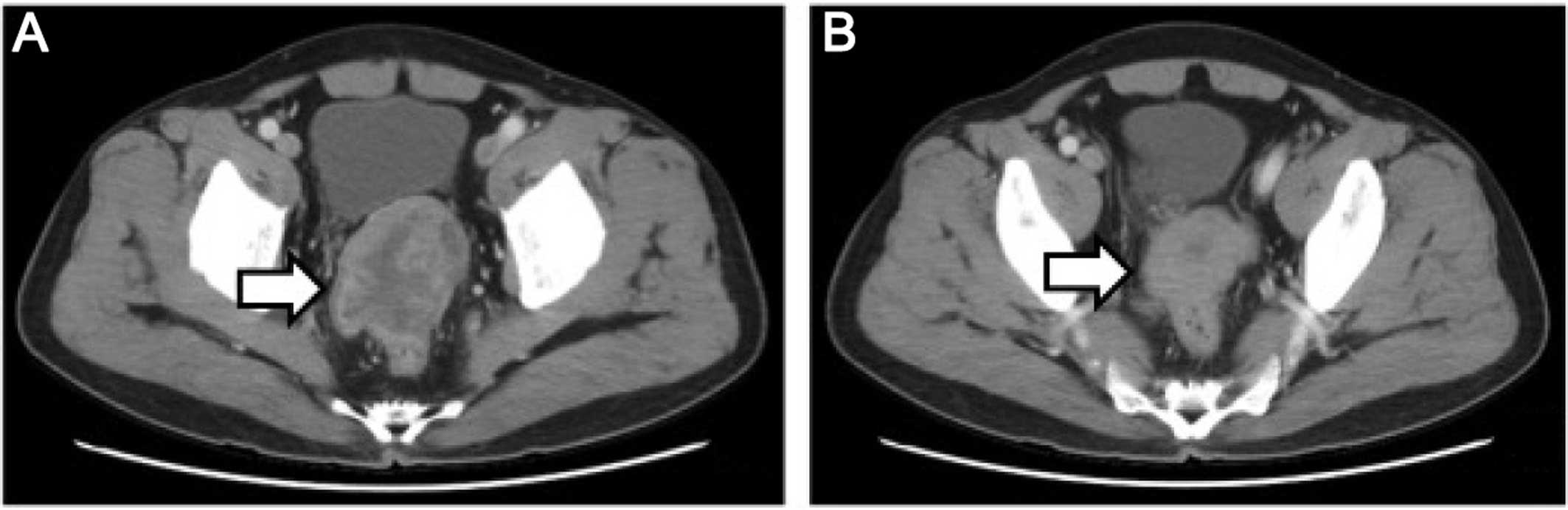
unresectable metastatic tumor was detected in the rectum by CT
examination (Fig. 4A). Subsequently,
the patient underwent three cycles of chemotherapy for the tumor
metastasis. The chemotherapy regimen included epirubicin 50 mg on
days 1 and 2; dacarbazine 200 mg daily on days 1–5; and 50 mg
docetaxel on day 1. The patient tolerated the chemotherapy well. In
a recent CT scan (November 15, 2015), the growth of the
retroperitoneal tumor, as well as that of the metastatic tumor in
the rectum, had been stabilized (Figs.
3B and 4B).
All procedures performed were in accordance with the
ethical standards of the Institutional Research Committees of the
participating institutions and informed consent was obtained from
the patient regarding the publication of the case details.
Discussion
IMT is a neoplasm of intermediate biological
potential, which is characterized by spindle cell proliferation
with an inflammatory infiltrate. IMT may also be referred to as
inflammatory pseudotumor, pseudosarcomatous myofibroblastic
proliferation, inflammatory sarcoma, plasma cell granuloma and
inflammatory myohistocytic proliferation (3). This disease typically arises in the
lung, and rarely in sites such as the retroperitoneum, pelvis,
head/neck and extremities (4,5).
The diagnosis of IMT is made on the basis of
histological analysis and there are no established decisive
criteria for differential diagnosis. Histologically, the lesion is
predominantly composed of myofibroblasts in a myxoid to collagenous
stroma admixed with inflammatory cells, characterized by
paucicellular to moderately cellular spindle- to stellate-shaped
cells, with an admixed inflammatory infiltrate of varying density
(6). The characteristics of this
disease vary between different sites, possibly indicating the
different phases of its development. The major characteristics
include a spectrum of myofibroblastic proliferation, often with a
vaguely storiform architecture, along with varying amounts of
inflammatory infiltrates, mainly consisting of lymphocytes,
histiocytes and plasma cells. The presence of scattered eosinophils
has also been reported in a number of cases, particularly in the
head of pancreatic IMTs (2).
Although initially IMT was considered to be a benign
reactive lesion, this hypothesis has been challenged due to the
neoplastic characteristics in the clinical presentation, with a
high recurrence rate of 18–40%, the presence of regional
metastasis, and the acquired clonal chromosomal abnormality in the
cytogenetic analysis (7). Of all the
chromosomal aberrations identified in the disease, the chromosomal
region 2p23 near or within the ALK gene is consistently involved in
IMT (8). Rearrangements involving the
ALK locus on chromosome 2p23 have been documented in ~50% of IMTs
(6). Distant metastases occur
primarily in ALK-negative IMTs, but local recurrence occurs
regardless of ALK expression (4). In
the present case, the specimen stained negative for ALK and rectal
metastasis occurred shortly after surgical removal of the primary
tumor.
The management of IMTs may be challenging, as there
are currently no established treatment protocols; radical local
excision of the tumor is the mainstay of treatment. IMTs occurring
in the abdomen or retroperitoneum often have a propensity for more
aggressive behavior with multiple recurrences, invasion into
adjacent structures and metastases, making curative resection
impossible and promoting local recurrence (4). In our patient, the first attempt of
radical excision of the tumor was unsuccessful due to its proximity
to the left renal artery, resulting in a positive margin, which led
to tumor recurrence. Adjuvant therapy would be indicated in this
case; however, a standardized guideline for chemotherapy and
radiotherapy is not available, due to the rarity of the disease.
Although the progression of the primary mass was controlled by
CT-guided radiofrequency ablation, a distant metastasis to the
rectum made further management necessary; chemotherapy was the
treatment of choice for this patient.
In the published literature, multiple agents have
been investigated in the treatment of malignant IMTs, including
vincristine, etoposide, cisplatin, adriamycin and methotrexate
(9–11). Non-steroidal anti-inflammatory drugs
have also been added to the regimens, with or without
chemotherapeutic agents (12).
However, the clinical outcomes are difficult to interpret due to
the heterogeneity of the treatment modalities. Although novel
therapies have been reported for the treatment of the aggressive
form of IMT, including anti-inflammatory agents, antitumor necrosis
factor-α-binding antibodies (13)
and, more recently, the ATP-competitive inhibitors of ALK,
crizotinib, ceritinib and alectinib (14,15),
further application of these agents in clinical practice relies on
future clinical trials. Based on extrapolation from studies on soft
tissue malignancies and the National Comprehensive Cancer Network
recommendations (16), an aggressive
regimen was applied in the present case, using a combination of
epirubicin, dacarbazine and docetaxel, for the management of the
retroperitoneal IMT. After three cycles of chemotherapy, the result
is considered encouraging and the growth of the tumor appears to
have been arrested.
To the best of our knowledge, this is the only
published case report on the management of an aggressive
retroperitoneal IMT with epirubicin, dacarbazine and docetaxel
combination chemotherapy achieving a partial response in the
primary as well as the metastatic site. Although the results are
encouraging, the long-term prognosis of this patient is uncertain,
since the tumor has not been completely eliminated. Although a
third surgery attempting complete resection of the tumor may be an
option, given the nature of this disease, the long-term survival
prediction is not favorable due to the propensity of the lesion for
invasion and metastasis. Moreover, a laparotomy after chemotherapy
would be challenging and decision making regarding surgery depends
on the skill and experience of the surgical team, as well as on the
patient's overall condition. In the future, thorough investigation
of the heterogeneous nature of this disease and subsequent
development of targeted therapies may result in the successful
management of malignant IMTs.
References
|
1
|
Coffin CM, Dehner LP and Meis-Kindblom JM:
Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma and
related lesions: An historical review with differential diagnostic
considerations. Semin Diagn Pathol. 15:102–110. 1998.PubMed/NCBI
|
|
2
|
Pungpapong S, Geiger XJ and Raimondo M:
Inflammatory myofibroblastic tumor presenting as a pancreatic mass:
A case report and review of the literature. JOP. 5:360–367.
2004.PubMed/NCBI
|
|
3
|
Yamamoto H, Watanabe K, Nagata M, Tasaki
K, Honda I, Watanabe S, Soda H and Takenouti T: Inflammatory
myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary
Pancreat Surg. 9:116–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziadi S, Trimeche M, Mestiri S, Boujelbene
N, Mokni M, Sriha B and Korbi S: Retroperitoneal myofibroblastic
inflammatory tumor. Tunis Med. 89:400–401. 2011.PubMed/NCBI
|
|
6
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertocchini A, Lo Zupone C, Callea F,
Gennari F, Serra A, Monti L, de Ville de and Goyet J: Unresectable
multifocal omental and peritoneal inflammatory myofibroblastic
tumor in a child: Revisiting the role of adjuvant therapy. J
Pediatr Surg. 46:e17–e21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffin CA, Hawkins AL, Dvorak C, Henkle
C, Ellingham T and Perlman EJ: Recurrent involvement of 2p23 in
inflammatory myofibroblastic tumors. Cancer Res. 59:2776–2780.
1999.PubMed/NCBI
|
|
9
|
Dishop MK, Warner BW, Dehner LP, Kriss VM,
Greenwood MF, Geil JD and Moscow JA: Successful treatment of
inflammatory myofibroblastic tumor with malignant transformation by
surgical resection and chemotherapy. J Pediatr Hematol Oncol.
25:153–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Firat O, Ozturk S, Akalin T and Coker A:
Inflammatory myofibroblastic tumour. Can J Surg. 52:E60–E61.
2009.PubMed/NCBI
|
|
11
|
Tao YL, Wang ZJ, Han JG and Wei P:
Inflammatory myofibroblastic tumor successfully treated with
chemotherapy and nonsteroidals: A case report. World J
Gastroenterol. 18:7100–7103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Przkora R, Bolder U, Schwarz S, Jauch KW,
Spes J, Andreesen R and Mackensen A: Regression of nonresectable
inflammatory myofibroblastic tumours after treatment with
nonsteroidal anti-inflammatory drugs. Eur J Clin Invest.
34:320–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berger A, Kim C, Hagstrom N and Ferrer F:
Successful preoperative treatment of pediatric bladder inflammatory
myofibroblastic tumor with anti-inflammatory therapy. Urology.
70(372): e13–e15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katayama R, Lovly CM and Shaw AT:
Therapeutic targeting of anaplastic lymphoma kinase in lung cancer:
A paradigm for precision cancer medicine. Clin Cancer Res.
21:2227–2235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Casper ES, Conrad EU III, DeLaney TF, Ganjoo KN,
George S, et al: Gastrointestinal stromal tumors, version 2.2014. J
Natl Compr Canc Netw. 12:853–862. 2014.PubMed/NCBI
|