Introduction
In breast cancer, a common treatment for achieving
local control is for the patient to undergo mastectomy in order to
remove any detectable macroscopic disease. In early-stage disease,
this aims to remove the tumour and, therefore, reduce the incidence
of metastasis. However, mastectomy is not always able to remove all
disease foci, which may remain in the locoregional tissue. This may
lead to locoregional recurrence (LRR) and, subsequently, in some
cases, death from breast cancer. Radiotherapy, when used as an
adjuvant therapy, has the potential to remove small disease foci,
thereby reducing the risk of LRR.
The use of post-mastectomy radiotherapy (PMRT) has
long been established in the treatment of patients with T3/4 breast
cancer and/or those with ≥4 positive axillary lymph nodes, having
been associated with a clear survival benefit and reduction in
local recurrence, evidence that has reached a level of 1a (1). Therefore, its use in such patients is
currently recommended by several national bodies, including the
National Institute for Health and Clinical Excellence (2). The central issue currently is the role
of PMRT in intermediate-risk patients, meaning those with 1–3
positive lymph nodes. A meta-analysis published in 2014
demonstrated that the beneficial effects of PMRT remained apparent
in such patients, who received the same benefit as those with more
positive nodes (3), although no
additional benefit was observed in those without positive
nodes.
The aim of the present meta-analysis was to build
upon the evidence presented previously by focusing on the effect of
PMRT on overall survival (OS) and LRR in patients with 1–3 positive
axillary lymph nodes, regardless of the use of systemic therapy, by
including data from more recent studies.
Materials and methods
Types of studies and participants
Prospective clinical trials and retrospective case
series with reported outcomes as a function of PMRT in breast
cancer patients with 1–3 positive axillary lymph nodes were
considered. All the selected studies included female adult patients
with primary breast cancer and positive metastases to 1–3 axillary
lymph nodes, and all the patients were treated with mastectomy,
with or without PMRT.
Outcome measures
The primary outcome was OS in patients treated with
PMRT in the setting of primary breast cancer. The secondary
endpoint was LRR, when reported.
Search methods
A computer-aided search through the PubMed and Ovid
databases was performed to identify relevant literature. The lower
limit date for the search was set at 01/04/2015, with no upper
limit. The following search terms were used: ‘post-mastectomy
radiotherapy 1–3 lymph nodes survival’, ‘post-mastectomy 1–3 lymph
nodes’, ‘radiotherapy post-mastectomy <3 lymph nodes’ and
‘post-mastectomy’. The related articles function on PubMed was also
utilised and the bibliographies of relevant articles were analysed
in order to identify all relevant literature.
Data collection and analysis
The authors independently performed the study
selection according to the inclusion criteria outlined above.
Studies in full text were selected if they reported: i) either OS,
or LRR, or both, for adult female breast cancer patients who were
treated with mastectomy and PMRT compared with patients undergoing
mastectomy without PMRT in the presence of 1–3 positive axillary
lymph nodes; and ii) full text was available for data extraction.
The exclusion criteria were: i) studies that did not report OS or
LRR; and ii) case reports, commentaries, letters or reviews.
Data extraction
The authors extracted data independently using the
following items: characteristics of included studies (author,
publication date, study design, participants and interventions),
median age of the participants and the aforementioned outcomes.
Measure of treatment effect and
statistical analysis
Percentages and their 95% confidence intervals (CIs)
for OS and/or LRR as a function of the use of PMRT in patients with
1–3 positive lymph nodes were retrieved from each included study. A
meta-analysis of each outcome was then performed, following
assessment for heterogeneity using Cochrane's Q and I2 tests. The
results of these tests, plus a zero-effect test, determined the use
of either a fixed-effects or random-effects model. Potential
publication biases were evaluated with funnel plots for OS and LRR
in order to examine the relative symmetry of individual study
estimates around the overall estimate in addition to Duval and
Tweedie's trim and fill method. This was accompanied by Begg's and
Egger's tests. P<0.05 was considered to indicate statistically
significant differences.
The results were reported as a classic forest plot,
one for each outcome of OS and LRR. All the statistical analyses
were performed using RevMan 5.1 and Comprehensive Meta-Analysis,
version 2 software (Comprehensive Meta-Analysis Software, Englewood
NJ, USA).
Results
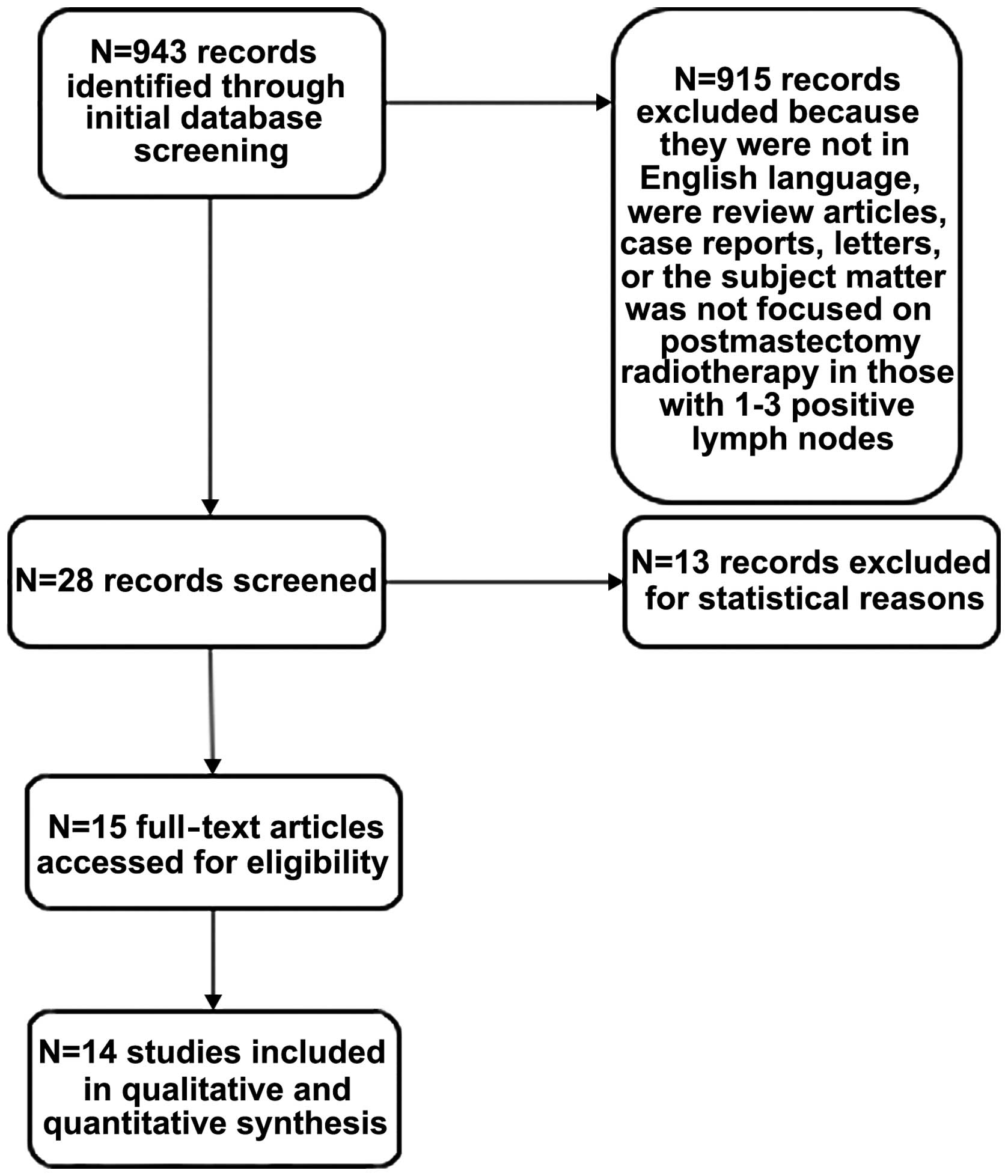
A total of 943 publications were identified, 14 of
which were included in this review (Tables I and IV), incorporating a total of 8,544
patients. The flow diagram of the study selection process is shown
in Fig. 1. A total of 13 studies were
excluded, as they did not include reports of either OS or LRR rates
as part of their results. All included studies were retrospective
case series. The primary endpoints of either OS or LRR rate, along
with 95% CIs were reported, or could be calculated for all the
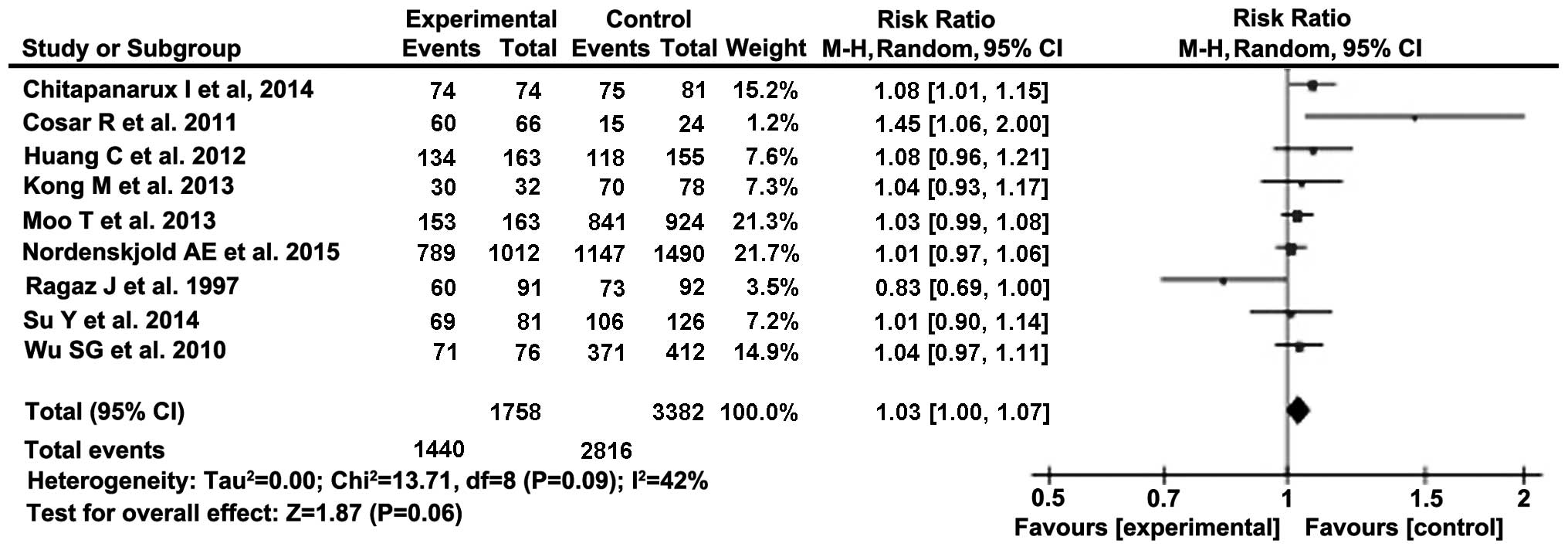
studies included. The pooled relative risk ratio (RR) for OS was
1.03 (95% CI: 1.00–1.07) and for LRR it was 0.30 (95% CI:
0.23–0.38), showing a benefit in delivering PMRT to patients with
1–3 positive lymph nodes.
 | Table I.Studies included in the OS analysis of
the effect of PMRT on breast cancer patients with 1–3 positive
lymph nodes. |
Table I.
Studies included in the OS analysis of
the effect of PMRT on breast cancer patients with 1–3 positive
lymph nodes.
| First author, year
(Refs.) | Country | Study design | Number of
participants (total) | Number in control
group | Number in
intervention group | Type of surgery | Median age
(years) | Median follow-up time
(months) | Overall survival in
controls (%) | Overall survival in
intervention group (%) | P-value |
|---|
| Nordenskjӧld, 2015
(4) | Sweden | Population-based | 2,502 | 1,490 | 1,012 | Mastectomy | NR | NR | 77.0 | 78.0 | 0.12 |
| Kong, 2013 (5) | Korea | Retrospective case
series | 110 |
78 |
32 | Mastectomy | 48.6 | 84 | 90.3 | 93.1 | 0.824 |
| Chitapanarux, 2014
(6) | Thailand | Retrospective case
series | 155 |
81 |
74 | Mastectomy | NR | 53.4 | 93.1 | 100.0 | 0.044 |
| Cosar, 2011 (7) | Turkey | Retrospective case
series |
90 |
24 |
66 | Mastectomy | 51 | 72 | 61.9 | 90.2 | 0.087 |
| Wu, 2010 (8) | China | Retrospective case
series | 488 | 412 |
76 | Mastectomy | 47 | 54 | 90.0 | 93.8 | 0.251 |
| Ragaz, 1997 (9) | Canada | Randomised controlled
trial | 183 |
92 |
91 | Mastectomy | NR | 150 | 79.0 | 66.0 | 0.06 |
| Su, 2014 (10) | Taiwan | Retrospective case
series | 207 | 126 |
81 | Mastectomy | 50 | 59.5 | 83.8 | 85.6 | 0.92 |
| Moo, 2013 (11) | USA | Retrospective case
series | 1,087 | 924 | 163 | Mastectomy | NR | 84 | 91.0 | 94.0 | 0.28 |
| Huang, 2012 (12) | China | Retrospective case
series | 318 | 155 | 163 | Mastectomy | 48.5 (mean) | 102 | 76.1 | 82.1 | 0.239 |
 | Table IV.Studies included in the locoregional
recurrence analysis of the effect of PMRT on breast cancer patients
with 1–3 positive lymph nodes. |
Table IV.
Studies included in the locoregional
recurrence analysis of the effect of PMRT on breast cancer patients
with 1–3 positive lymph nodes.
| First author, year
(Refs.) | Country | Study design | Number of
participants (total) | Number in control
group | Number in
intervention group | Type of
surgery | Median age
(years) | Median follow-up
(months) | Locoregional
recurrence rate, control group, n (%) | Locoregional
recurrence rate, intervention group, n (%) |
|---|
| Kong, 2013
(5) | Korea | Retrospective case
series |
110 | 78 | 32 | Mastectomy | 48.6 | 84 | 10 (12.8) | 2 (6.2) |
| Cosar, 2011
(7) | Turkey | Retrospective case
series |
90 | 24 | 66 | Mastectomy | 51 | 72 | 4 (17) | 2 (3.0) |
| Ragaz,1997
(9) | Canada | Randomised
controlled trial |
183 | 92 | 91 | Mastectomy | NR | 150 | 15 (16.0) | 6 (7.0) |
| Su, 2014 (10) | Taiwan | Retrospective case
series |
207 | 126 | 81 | Mastectomy | 50 | 59.5 | 15 (11.8) | 4 (4.7) |
| Moo, 2013 (11) | USA | Retrospective case
series | 1,087 | 924 | 163 | Mastectomy | NR | 84 | 40 (4.3) | 5 (3.2) |
| Huang, 2012
(12) | China | Retrospective case
series |
318 | 155 | 163 | Mastectomy | 48.5 (mean) | 102 | 17 (11.0) | 5 (3.1) |
| He, 2015 (13) | China | Retrospective case
series |
697 | 618 | 79 | Mastectomy | NR | 65 | 65 (10.5) | 1 (1.3) |
| Tendulkar, 2012
(14) | America | Retrospective case
series |
369 | 271 | 98 | Mastectomy | 56 | 62.4 | 24 (8.9) | 0 (0.0) |
| McBride, 2014
(15) | USA | Retrospective case
series | 1,027 | 800 | 235 | Mastectomy | NR | 144.5 | 71 (8.9) | 11 (4.7) |
| Harris, 2013
(16) | USA | Retrospective case
series |
250 | 204 | 46 | Mastectomy | NR | 65.6 | 13 (6.4) | 1 (2.2) |
| Overgaard, 1997
(17) | Denmark | Randomised
controlled trial | 1,061 | 516 | 545 | Mastectomy | NR | 114 | 155 (30.0) | 38 (7.0) |
OS
For OS, a total of 9 studies were included (Table I), incorporating 5,837 patients with a
mean follow-up of 80.4 months (range, 53.4–150 months). Information
collected included total participants in the treatment and control
arms and respective OS rates. The mean follow-up time was 80.4
months. First, heterogeneity was assessed according to Cochran's Q
and I2 tests. Cochran's Q test suggested that the null hypothesis
(that the treatment effect would be equal to 0) could be rejected,
whilst the I2 was calculated at 42%, indicating moderate
heterogeneity (Table II). To account
for this heterogeneity, we calculated summary statistics using the
random-effects model.
 | Table II.Heterogeneity tests for overall
survival. |
Table II.
Heterogeneity tests for overall
survival.
| Testa | Null vs.
alternative/thresholds | Measure | Df | χ2 | Prob level |
|---|
| Cochran's Q |
H0: all studies are
evaluating the same effect | Risk ratio | 8 |
χ2=13.7 | 0.089928 |
|
|
Ha: not all studies are
evaluating the same effect |
|
|
|
|
| I2 | 0 to 40%: may not
be important | Risk ratio |
|
I2=42 |
|
|
| 30 to 60%: may
represent moderate heterogeneity |
|
|
|
|
|
| 50 to 90%: may
represent substantial heterogeneity |
|
|
|
|
|
| 75 to 100%:
Indicates considerable heterogeneity |
|
|
|
|
RRs were calculated from the results of the studies
listed in Fig. 2. The summary RR was
then calculated as 1.03 (95% CI: 1.00–1.07). Therefore, according
to the summary effect, OS is 3% higher following PMRT. When the
relative risk measure value is equal to 1.00, it indicates no
difference in OS between intervention and control groups. As the
lower limit of the 95% CI is 1.00, an additional zero-effect test
was performed, based on the natural logarithms of RRs (Table III).
 | Table III.Zero-effect tests. |
Table III.
Zero-effect tests.
| Testa | Null vs.
alternative | Measure | Df | χ2 | Prob level |
|---|
|
Non-directional |
H0: all treatment
effects are zero | ln (RR) | 9 | 20.06 | 0.017546 |
|
|
Ha: at least one is not
zero |
|
|
|
|
| Directional |
H0: all treatment
effects are zero | ln (RR) | 1 |
6.86 | 0.008815 |
|
|
Ha: effects are equal to
the same non-zero quantity |
|
|
|
|
Both tests achieved statistical significance (set at
P<0.05) at the 5% significance level; therefore, the null
hypothesis can be rejected. Furthermore, we may conclude that PMRT
exerts a small but positive effect on the OS of patients.
The last item in our analysis was to estimate the
publication bias of the included studies by incorporating them into
a funnel plot. Within the funnel plot, not all studies are within
the 95% CI, and it is not conclusive whether all the studies are
symmetrical around the combined effect size, indicating absence of
publication bias. Using Duval and Tweedie's trim and fill method
imputes an allegedly omitted study with a natural logarithm RR of
0.35. The recomputed combined effect estimate remains very close to
our initial estimate of 1.03 on the random-effects model: 1.03 (95%
CI: 0.99–1.07) vs. 1.03 (95% CI: 1.00–1.07), respectively. The Begg
and Mazumdar rank correlation test also supported the absence of
publication bias, showing no correlation between the study size and
the effect size. In conclusion, we may support our estimate of a
summary RR=1.03, with a 95% CI of 1.00–1.07.
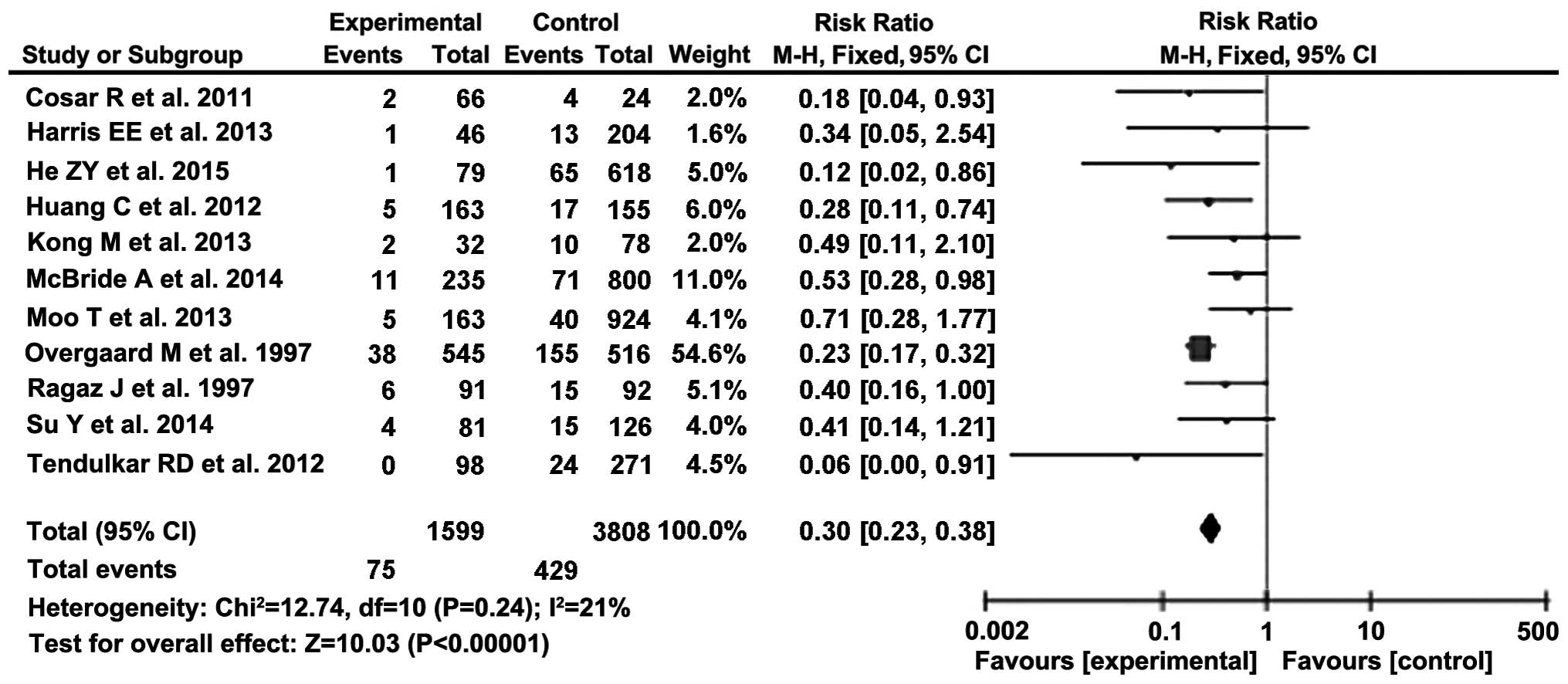
LRR
The effect of PMRT on LRR was also analysed using 11
studies (5,7,9–17), incorporating 5,399 patients (Fig. 3, Table
IV). The mean follow-up time was calculated as 91.2 months
(range, 59.5–150 months). The effect of the intervention was again
estimated using the RR measure. We used the zero-effect test,
Cochrane's Q and I2 tests for heterogeneity, summary effect using a
forest plot, and checked for publication bias using a funnel plot,
Egger's test of the intercept, and Begg and Mazumdar rank
correlation test.
Using the zero-effect test, we were able to reject
the null hypothesis, in which the effect is equal to 0,
corresponding to a RR of 1. Hence, it may be predicted that PMRT
should exert a statistically significant effect on LRR (Table V).
 | Table V.Zero-effect test rejecting the null
hypothesis. |
Table V.
Zero-effect test rejecting the null
hypothesis.
| Testa | Null vs.
alternative | Measure | Df | χ2 | Prob level |
|---|
|
Non-directional |
H0: all treatment
effects are zero | ln (RR) | 11 | 106.11 | 0.0000 |
|
|
Ha: at least one is not
zero |
|
|
|
|
| Directional |
H0: all treatment
effects are zero | ln (RR) | 1 |
93.46 | 0.0000 |
|
|
Ha: effects are equal to
the same non-zero quantity |
|
|
|
|
Cochran's Q and I2 tests suggest that all the
studies are evaluating the same effect, and heterogeneity is not
significantly present (Table VI).
Therefore, the fixed-effects model may be used to estimate the
combined effect.
 | Table VI.Tests of heterogeneity showing
non-significant heterogeneity and that all studies are evaluating
the same effect (fixed-effects model). |
Table VI.
Tests of heterogeneity showing
non-significant heterogeneity and that all studies are evaluating
the same effect (fixed-effects model).
| Testa | Null vs.
alternative/thresholds | Measure | Df | χ2 | Prob level |
|---|
| Cochran's Q |
H0: all studies are
evaluating the same effect | Risk ratio | 10 |
χ2=12.7 | 0.240932 |
|
|
Ha: not all studies are
evaluating the same effect |
|
|
|
|
| I2 | 0 to 40%: May not
be important | Risk ratio |
| I2=21% |
|
|
| 30 to 60%: May
represent moderate heterogeneity |
|
|
|
|
|
| 50 to 90%: May
represent substantial heterogeneity |
|
|
|
|
|
| 75 to 100%:
Indicates considerable heterogeneity |
|
|
|
|
The summary effect was calculated using a
fixed-effects model, which was incorporated into a forest plot
(Fig. 3). The combined RR of the
effect of PMRT on LRR was calculated as 0.30 (95% CI: 0.23–0.38),
indicating that PMRT considerably decreases the risk of LRR.
To check for publication bias, a funnel plot was
created using Duval and Tweedie's trim and fill method.
Additionally, Egger's test and Begg and Mazumdar rank correlation
test were performed. On the funnel plot, all estimates were within
the 95% CI and were placed relatively symmetrically around the
combined effect, indicating no publication bias. Duval and
Tweedie's trim and fill method did not signify any missing study,
generating an unchanged estimate of the combined RR. the results of
the Egger's test and Begg and Mazumdar tests are outlined in
Table VII. These concluded that
there was no publication bias. Therefore, we may support the
estimate of a summary RR of 0.30 (95% CI: 0.23–0.38), indicating
that PMRT significantly reduces the risk of LRR in breast
cancer.
 | Table VII.Tests for publication bias indicating
that there was no sign of any missing study and, therefore, the
estimate of the combined risk ratio remained unchanged (no
publication bias). |
Table VII.
Tests for publication bias indicating
that there was no sign of any missing study and, therefore, the
estimate of the combined risk ratio remained unchanged (no
publication bias).
| Testa | Results |
|---|
| Egger's test of the
intercept | Intercept (B0) is
0.22971, 95% confidence interval (−1.19571, 1.65513), with
t=0.36455, degree of freedom = 9. The 1-tailed P-value
(recommended) is 0.36193, and the 2-tailed P-value is 0.72387 |
| Begg and mazumdar
rank correlation test | Kendall's tau b
(corrected for ties, if any) is −0.32727, with a 1-tailed P-value
(recommended) of 0.08056 or a 2-tailed P-value of 0.16112 (based on
continuity-corrected normal approximation) |
Overall results of the
meta-analysis
Overall, the results of the meta-analysis
demonstrated that PMRT significantly decreased the risk of LRR
(RR=0.30, 95% CI: 0.23–0.38), whereas there was a non-significant
increase in OS (RR=1.03, 95% CI: 1.00–1.07).
Discussion
The meta-analysis results demonstrated that PMRT
appears to significantly reduce the risk of LRR, with a minor
benefit in terms of OS. These results, therefore, support the use
of PMRT to reduce LRR in breast cancer patients, with a small
chance of increasing OS. The small, non-significant benefit in OS
through using PMRT may be explained by the follow-up times used in
the studies analysed. The mean follow-up time was 80.4 months
(range, 53.4–150 months). A longer follow-up time may allow the
significant benefit seen in LRR to translate to an increased
benefit in OS. Additionally, a number of other risk factors, which
were not included in the present study, may also affect these
outcomes. Tumour factors, such as receptor status and size, have
also been found to affect both LRR and OS. Other information, which
may exert an effect on OS, includes comorbidities and patient
age.
In this meta-analysis, we did not control for any
additional factors that may also have an impact on OS and LRR
rates. However, some of the studies that were included performed
multivariate analyses in order to identify any independent
prognostic factors. In terms of OS, younger age, medial tumour
location, Her2/neu overexpression and negative oestrogen receptor
status were associated with poorer outcomes and a reduction in OS
(4–6,10,12). Higher-grade disease, a triple-negative
subtype, age <40 years, Her2/neu overexpression and negative
oestrogen receptor status were all identified as independent poor
prognostic factors for LRR (5,6,10,12,13). In
order to fully elucidate the effect of these factors, and other
factors, such as the use of systemic therapy, on the suggested LRR
rates and OS when PMRT is used, future prospective randomised
trials are warranted. This may enable the identification of a
subgroup of patients for whom PMRT may be particularly beneficial,
in terms of reducing LRR and increasing OS.
The Early Breast Cancer Trialists' Collaborative
Group recently conducted a meta-analysis investigating the effect
of PMRT on 10-year recurrence and 20-year breast cancer mortality.
Although not reporting specifically on women with only 1–3 positive
lymph nodes, they did report on the effect of PMRT on LRR within
this group, and demonstrated that PMRT significantly reduced the
risk of LRR, as well as overall recurrence and breast cancer
mortality (3). However, due to the
long follow-up used in this trial, the patients included were
treated a long time ago; therefore, systemic therapy, which was
used additionally, would not have been as effective as the systemic
therapy currently used. Therefore, the benefits exclusively from
PMRT in modern trials are likely to be smaller, due to the use of
modern systemic therapy, such as Herceptin, endocrine therapy and
improved chemotherapy regimens.
One of the main issues with PMRT is the risk of
cardiac toxicity caused by chest wall irradiation. Cardiac
irradiation has been associated with significant pathological
damage to the heart, such as microcirculatory damage, which may
lead to ischaemia, fibrosis, accelerated atherosclerosis,
pericardial effusion and pericardial thickening (18). Earlier studies associated PMRT with
adverse cardiac effects, such as myocardial infarctions, and a
significantly increased number of cardiac deaths, with the left
anterior descending coronary artery suggested to be particularly
vulnerable to damage (19). Despite
this, more recent studies have dispelled these findings, with one
prospective trial showing no clinically significant acute or late
cardiac adverse events at the 2-year follow-up, and no difference
in left ventricular ejection fraction (18). Although this particular trial had a
short follow-up time, the reduction in the risk of adverse cardiac
events has been attributed to the advancements in radiotherapy
techniques. For example, the use of three-dimensional computed
tomography-guided planning in order to minimise the exposure of the
heart has reduced the effects of late cardiac toxicity (19,20). In a
study by Doyle et al, it was reported that the use of
radiotherapy did not increase the risk of myocardial infarction
over a period of 10 years (21).
Subsequently, it may be concluded that, with proper planning, PMRT
does not necessarily increase the risk of cardiac adverse effects,
although it would be useful to conduct a trial assessing this risk
in patients with 1–3 positive lymph nodes, in order to fully
establish whether the benefits regarding LRR incidence outweigh any
risks to the heart.
In addition, the adverse effects of PMRT on breast
reconstruction should be considered in the benefit-risk analysis in
the context of LRR incidence and OS benefits. PMRT increases the
complication rate of any type of reconstruction, autologous or
implant-based. Most guidelines also suggest it is better to delay
reconstruction if it is known preoperatively that radiotherapy will
be required. However, immediate reconstruction is associated with a
better quality of life and reduces the risks of undergoing a second
surgery (22). Despite this, it has
been suggested that radiotherapy performed after reconstruction may
lead to a higher complication rate than if reconstruction is
delayed. In a previous study investigating the use of adjuvant
radiotherapy in porcine acellular dermis-assisted breast
reconstruction, the rate of total complications and
implant/expander loss was significantly higher in irradiated
breasts (23). Likewise, a study
investigating immediate autologous reconstruction found that there
was an increased risk of fat necrosis when the breasts were
irradiated (24). Therefore, it is
important to take this into account when planning the care of
patients who are likely to require radiotherapy, in order to
minimise the complications and optimise the aesthetic outcome.
There were a number of limitations to this
meta-analysis. All the studies included were retrospective case
series, whereas, ideally, prospective randomised trials would be
useful in order to increase the reliability of the results.
Additionally, a relatively limited number of studies were included
in each section. Despite this, many of the studies were published
in the last 5 years, indicating that this is a growing area of
research; therefore, future analyses may be able to draw their
conclusions from a significantly larger pool of research. For
example, the SUPREMO trial in the UK, which is currently being
undertaken, aims to determine the effect of PMRT on OS in women at
intermediate risk of LRR. However, the results will not be
available for a number of years, due to a minimal 10-year follow-up
(25).
In conclusion, PMRT in women with breast cancer with
1–3 positive lymph nodes results is associated with a significant
decrease in LRR and a relatively small OS benefit. In view of the
fact that the OS benefit is relatively small at 3%, it would be
reasonable to recommend PMRT to a selected group of patients with
other risk factors, such as young age, oestrogen receptor-negative,
Her2-positive, large, poorly differentiated tumours, following
detailed multidisciplinary discussion until the results of ongoing,
large-scale randomised controlled trials become known. In light of
the risk of cardiac toxicity, the threshold for recommending PMRT
will be lower for tumours of the right breast, where there is a
lower risk of adverse cardiac effects. The results of this
meta-analysis may enhance the informed consent process for PMRT in
breast cancer patients with 1–3 positive nodes.
References
|
1
|
Wenz F, Sperk E, Budach W, Dunst J, Feyer
P, Fietkau R, Haase W, Harms W, Piroth MD, SautterBihl ML, et al:
Breast Cancer Expert Panel of the German Society of Radiation
Oncology (DEGRO): DEGRO practical guidelines for radiotherapy of
breast cancer IV: Radiotherapy following mastectomy for invasive
breast cancer. Strahlenther Onkol. 190:705–714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dewis R and Gribbin J: Breast Cancer:
Diagnosis and treatment. An assessment of needNational Institute
for Health and Clinical Excellence clinical guidelines. National
Collaborating Centre for Cancer; Cardiff (UK): February. 2009
|
|
3
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group). McGale P, Taylor C, Correa C, Cutter D, Duane
F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of radiotherapy
after mastectomy and axillary surgery on 10-year recurrence and
20-year breast cancer mortality: Meta-analysis of individual
patient data for 8135 women in 22 randomised trials. Lancet.
383:2127–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordenskjöld AE, Fohlin H, Albertsson P,
Arnesson LG, Chamalidou C, Einbeigi Z, Holmberg E, Nordenskjöld B
and Karlsson P: Swedish Western and Southeastern Breast Cancer
Groups: No clear effect of postoperative radiotherapy on survival
of breast cancer patients with one to three positive nodes: A
population-based study. Ann Oncol. 26:1149–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong M and Hong SE: Which patients might
benefit from postmastectomy radiotherapy in breast cancer patients
with t1-2 tumor and 1-3 axillary lymph nodes metastasis? Cancer Res
Treat. 45:103–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chitapanarux I, Tharavichitkul E,
Jakrabhandu S, Klunklin P, Onchan W, Srikawin J, Pukanhaphan N,
Traisathit P and Vongtama R: Real-world outcomes of postmastectomy
radiotherapy in breast cancer patients with 1-3 positive lymph
nodes: A retrospective study. J Radiat Res. 55:121–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cosar R, Uzal C, Tokatli F, Denizli B,
Saynak M, Turan N, Uzunoglu S, Ozen A, Sezer A, Ibis K, et al:
Postmastectomy irradiation in breast in breast cancer patients with
T1-2 and 1-3 positive axillary lymph nodes: Is there a role for
radiation therapy? Radiat Oncol. 6:282011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu SG, He ZY, Li FY, Wang JJ, Guo J, Lin Q
and Guan XX: The clinical value of adjuvant radiotherapy in
patients with early stage breast cancer with 1 to 3 positive lymph
nodes after mastectomy. Chin J Cancer. 29:668–676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ragaz J, Jackson SM, Le N, Plenderleith
IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM,
Paradis M, et al: Adjuvant radiotherapy and chemotherapy in
node-positive premenopausal women with breast cancer. N Engl J Med.
337:956–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su YL, Li SH, Chen YY, Chen HC, Tang Y,
Huang CH, Chou FF, Wu SC and Rau KM: Post-mastectomy radiotherapy
benefits subgroups of breast cancer patients with T1-2 tumor and
1-3 axillary lymph node(s) metastasis. Radiol Oncol. 48:314–322.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moo TA, McMillan R, Lee M, Stempel M,
Patil S, Ho A and El-Tamer M: Selection criteria for postmastectomy
radiotherapy in t1-t2 tumors with 1 to 3 positive lymph nodes. Ann
Surg Oncol. 20:3169–3174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang CJ, Hou MF, Chuang HY, Lian SL,
Huang MY, Chen FM, Fu OY and Lin SF: Comparison of clinical outcome
of breast cancer patients with T1-2 tumor and one to three positive
nodes with or without postmastectomy radiation therapy. Jpn J Clin
Oncol. 42:711–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He ZY, Wu SG, Zhou J, Li FY, Lin Q, Lin HX
and Sun JY: Postmastectomy radiotherapy improves disease-free
survival of high risk of locoregional recurrence breast cancer
patients with T1-2 and 1 to 3 positive nodes. PLoS One.
10:e01191052015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tendulkar RD, Rehman S, Shukla ME, Reddy
CA, Moore H, Budd GT, Dietz J, Crowe JP and Macklis R: Impact of
postmastectomy radiation on locoregional recurrence in breast
cancer patients with 1-3 positive lymph nodes treated with modern
systemic therapy. Int J Radiat Oncol Biol Phys. 83:e577–e581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McBride A, Allen P, Woodward W, Kim M,
Kuerer HM, Drinka EK, Sahin A, Strom EA, Buzdar A, Valero V, et al:
Locoregional recurrence risk for patients with T1,2 breast cancer
with 1-3 positive lymph nodes treated with mastectomy and systemic
treatment. Int J Radiat Oncol Biol Phys. 89:392–398. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harris EE, Freilich J, Lin HY, Chuong M
and Acs G: The impact of the size of nodal metastases on recurrence
risk in breast cancer patients with 1-3 positive axillary nodes
after mastectomy. Int J Radiat Oncol Biol Phys. 85:609–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overgaard M, Hansen PS, Overgaard J, Rose
C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen
MB and Zedeler K: Postoperative radiotherapy in high-risk
premenopausal women with breast cancer who receive adjuvant
chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N
Engl J Med. 337:949–955. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan M, Gupta M and Seam R: Analysis of
cardiac adverse events following postmastectomy hypofractionated
radiotherapy. Chin Clin Oncol. 3:472014.PubMed/NCBI
|
|
19
|
Kunkler IH: Radiotherapy of the regional
lymph nodes: Shooting at the sheriff? Breast. 18(Suppl 3):
S112–S120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris EE: Cardiac mortality and morbidity
after breast cancer treatment. Cancer Control. 15:120–129.
2008.PubMed/NCBI
|
|
21
|
Doyle JJ, Neugut AI, Jacobson JS, Wang J,
McBride R, Grann A, Grann VR and Hershman D: Radiation therapy,
cardiac risk factors and cardiac toxicity in early-stage breast
cancer patients. Int J Radiat Oncol Biol Phys. 68:82–93. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berbers J, van Baardwijk A, Houben R,
Heuts E, Smidt M, Keymeulen K, Bessems M, Tuinder S and Boersma LJ:
‘Reconstruction: Before or after postmastectomy radiotherapy?’ A
systematic review of the literature. Eur J Cancer. 50:2752–2762.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell RE: Porcine acellular
dermis-assisted breast reconstruction: Influence of adjuvant
radiotherapy on complications and outcomes. Plast Reconstr Surg
Glob Open. 1:e772013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rochlin DH, Jeong AR, Goldberg L, Harris
T, Mohan K, Seal S, Canner J and Sacks JM: Postmastectomy radiation
therapy and immediate autologous breast reconstruction: Integrating
perspectives from surgical oncology, radiation oncology, and
plastic and reconstructive surgery. J Surg Oncol. 111:251–257.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kunkler IH, Canney P, van Tienhoven G and
Russell NS: MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group:
Elucidating the role of chest wall irradiation in
‘intermediate-risk’ breast cancer: The MRC/EORTC SUPREMO trial.
Clin Oncol (R Coll Radiol). 20:31–34. 2008. View Article : Google Scholar : PubMed/NCBI
|