Introduction
Ovarian clear-cell adenocarcinoma (CCA) has been
recognized as a distinct entity among epithelial ovarian carcinomas
(EOCs) with respect to its chemoresistant nature and frequent
concurrence of endometriosis and clear-cell adenofibroma (CCAF). In
Western populations, CCAs account for ~5–13% of all EOCs, whereas
in Japan, its prevalence rises to 15–25% of all EOCs (1–3).
Approximately two thirds of CCAs were diagnosed at early-stage
disease; however, CCA has a relatively resistant phenotype to
platinum-based chemotherapy, resulting in extremely poor prognosis,
irrespective of surgical stages (4).
Therefore, it is essential to elucidate a novel therapeutic target
for CCA and to develop novel therapeutic strategies.
High-grade serous ovarian cancer, which is a major
subtype of all histological subtypes, is characterized by
TP53 mutations and frequent mutations or defects in
BRCA1/2 pathway. By contrast, CCA appears to harbor a
different molecular profile, including activating mutations in
PIK3CA, and loss of PTEN and ARID1A (5–9).
ARID1A mutation, in particular, is frequently observed in
endometriosis-associated ovarian clear-cell and endometrioid
adenocarcinoma, and it has been suggested that the mutation is as
an early molecular event in the development of
endometriosis-related CCA (8,9,10). These
distinct molecular features of CCA serve emphasis on the
requirement to develop subtype-specific therapeutic approachs in
the management of EOC.
Additionally, previous reports have suggested that
CCAs are classified into two distinct molecular subtypes and that
these subtypes have different clinical outcome (11). It was demonstrated that
endometriosis-related CCA and CCAF-related CCA had different
carcinogenic pathways (12,13). Certain previous reports suggested that
ARID1A somatic mutation and subsequent BAF250a protein loss
in CCA was correlated with response to chemotherapy and poor
prognosis (14); however, other
previous reports revealed no significance (10,15–17). To
date, the impact of BAF250a protein expression in response to
primary chemotherapy and the prognoses of CCAs has remains to be
determined.
The aim of the present study was to clarify whether
loss of BAF250a expression correlated with early tumorigenesis of
CCA, and to evaluate the significance of BAF250a-deficient
expression on clinicopathological variables in CCAs in a large
series of patients treated at a single institution.
Materials and methods
Patients
A total of 97 cases of CCA treated between 1984 and
2007 at the National Defense Medical College Hospital, (Tokorozawa,
Japan) were enrolled in the present study. Of the 97 CCAs, a
consecutive series of 38 CCAs synchronous with endometriosis
(EM-related CCAs) and 21 CCAs adjacent to CCAF component
(CCAF-related CCAs) were identified, according to the
histopathological criteria described previously (18). Of those, 31 non-atypical
endometrioses, 38 atypical endometrioses, 20 benign CCAFs and 21
borderline CCAFs were identified. A total of 18 cases with solitary
endometriosis that had no CCA were used as controls. All patients
provided written informed consent for the present study.
Immunohistochemical (IHC)
staining
Two core specimens, 1.5 mm in diameter, for each
case were obtained from cancer tissue blocks and transferred to
recipient blocks using a Tissue Microarrayer (Beecher Instrument,
Silver Spring, MD, USA). All specimens were cut into 4-µm-thick
slices to make tissue sections for IHC staining. The tissue
sections were deparaffinized and boiled in an autoclave at 121°C
for 15 min in 0.01 mol/l citrate buffer (pH 6.0) and were then
allowed to cool at room temperature. Endogenous peroxidase activity
was blocked using methanol added to 0.3% hydrogen peroxidase. The
slides were incubated at 4°C overnight with mouse monoclonal
primary antibody against BAF250a (cat. no. sc-32761; dilution,
1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Following incubation, the samples were reacted with dextran polymer
reagent combined with secondary antibodies and peroxidase (cat. no.
Z0420; 1:200; Dako A/S, Glostrup, Denmark) for 1 h at room
temperature. Specific antigen-antibody reactions were visualized
with 0.2% diaminobenzidine tetrahydrochrolide and hydrogen
peroxidase, and counterstaining was performed using Mayer's
hematoxylin. Non-neoplastic cells, including fibroblasts and
lymphocytes, served as positive internal controls. As negative
controls, tissue sections without the primary antibody were
used.
For BAF250a detection, the presence of nuclear
immunoreaction was taken into account for assignment of
immuno-positivity. The lesions were considered to be positive for
BAF250a if 50% or more of tumor cells in the area of interest
showed equal to or more strong immunoreactive intensity compared
with the positive controls (BAF250a-retained cases). If no
detectable nuclear staining of tumor cells or <50% of tumor
cells in the area of interest showed less immunoreactive intensity
compared with the positive controls, they were defined as having a
loss of BAF250a expression (BAF250a-deficient cases). The lesions
were assessed independently by two observers (Masafumi Kato and
Morikazu Miyamoto) in a blinded manner and any discrepancies
between the two observers were resolved by conferring over a
multi-viewer microscope.
Patient characteristics
Patient background, including age, concurrence of
endometriosis, co-existence of CCAF, international federation of
gynecology and obstetrics (FIGO) stage, residual tumor in primary
surgery and chemotherapy regimen were compared, according to the
BAF250a expression status. In addition, frequencies of loss of
BAF250a expression in EM-related CCAs and CCAF-related CCAs were
examined, as well as precursors (non-atypical endometrioses,
atypical endometrioses, benign CCAFs and borderline CCAFs). Tumor
response to adjuvant chemotherapy in evaluable cases,
progression-free survival and overall survival were analyzed
according to BAF250a expression status. Multivariate analyses for
overall survival and progression-free survival were performed.
Statistical analysis
Statistical analyses were performed using Stat Mate
IV software (ATMS, Tokyo, Japan) and Statview version 5 software
(SAS Institute Japan, Ltd., Tokyo, Japan). Student's t-test and
χ2 test were used to compare patient characteristics of
two groups. For survival analyses, Kaplan-Meier curves and the log
rank test were used. Prognostic significance was analyzed using the
Cox proportional hazard model using variables as follows: Age
(continuous variable), concurrence of endometriosis (yes vs. no),
FIGO stage (I/II vs. III/IV), residual tumor (≤1 cm vs. >1 cm)
and BAF250a status (BAF250a-deficient vs. BAF250a-retained).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between patient
characteristics and expression of BAF250a
The characteristics of the patients were assessed
according to the BAF250a expression status, and this is shown in
Table I. BAF250a-deficient expression
was observed in 30% (29/97) of all cases. No differences were
observed in age, FIGO stage, residual tumor in primary surgery and
chemotherapy regimen between the two groups. Concurrence of
endometriosis was observed more frequently in BAF250a-deficient
cases compared with in BAF250a-retained cases (P<0.05). By
contrast, co-existence of CCAF was significantly more frequent in
BAF250a-retained cases compared with that in BAF250a-deficient
cases (P=0.04).
 | Table I.Patient characteristics according to
the expression of BAF250a. |
Table I.
Patient characteristics according to
the expression of BAF250a.
| Characteristic | BAF250a-deficient
cases (n=29) | BAF250a-retained
cases (n=68) | P-value |
|---|
| Median age
(range) | 51 (36–67) | 52 (35–75) | 0.09 |
| Concurrence of
endometriosis |
|
| <0.05 |
| Yes | 20 (69%) | 32 (47%) |
|
| No | 9
(31%) | 36 (53%) |
|
| Co-existence of
CCAF |
|
| 0.04 |
| Yes | 2
(7%) | 19 (28%) |
|
| No | 27 (93%) | 49 (72%) |
|
| FIGO stage |
|
| 0.17 |
| Stage
I/II | 15 (52%) | 45 (66%) |
|
| Stage
III/IV | 14 (48%) | 23 (34%) |
|
| Residual tumor in
primary surgery |
|
| 0.14 |
| 0 cm | 15 (52%) | 42 (62%) |
|
| ≤1
cm | 4
(14%) | 12 (18%) |
|
| >1
cm | 10 (34%) | 14 (21%) |
|
| Chemotherapy
regimen |
|
| 0.85 |
|
Cyclophosphamide + adriamycin
+ cisplatin | 12 (41%) | 27 (40%) |
|
|
Irinotecan + cisplatin | 8
(28%) | 24 (35%) |
|
|
Paclitaxel +
carboplatin/cisplatin | 3
(10%) | 7
(10%) |
|
|
None/unknown | 6
(21%) | 10 (15%) |
|
Frequency of BAF250a expression
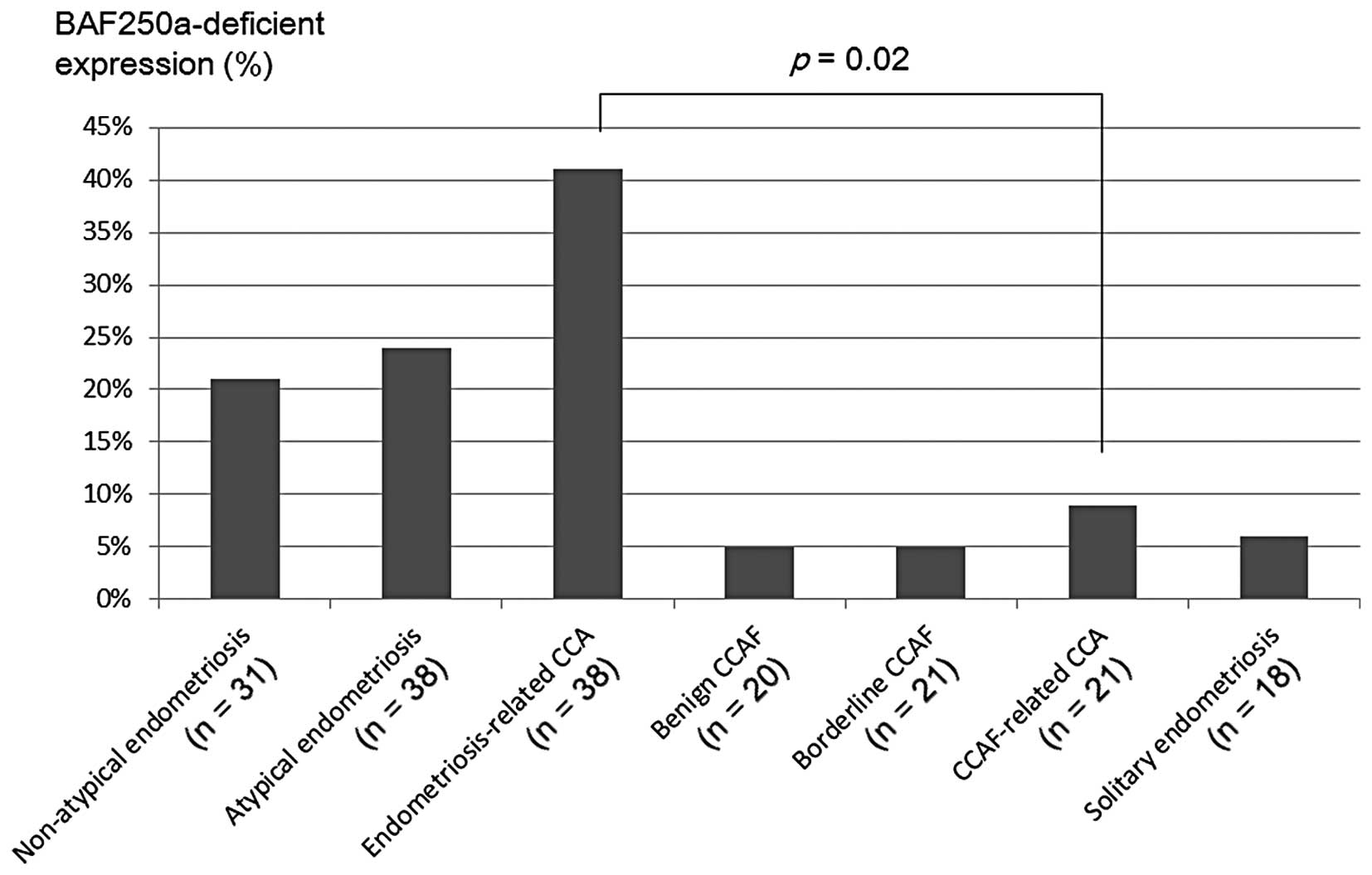
The frequencies of BAF250a-deficient expression were
19% (6/31) in non-atypical endometriosis, 26% (10/38) in atypical
endometriosis, 5% (1/20) in benign CCAF, 5% (1/21) in borderline
CCAF, 39% (15/38) in EM-related CCA and 10% (2/21) in CCAF-related
CCA (Fig. 1). In solitary
endometriosis, loss of BAF250a expression was detected in 6% (1/18)
of cases. In comparison with the frequency of BAF250a-deficient
expression between EM-related CCAs and CCAF-related CCAs, a
significant difference was observed between the two groups
(P=0.02).
Response rate of chemotherapy
The response rate of chemotherapy in evaluable cases
is shown in Table II. No significant
difference was observed in response rate of primary chemotherapy
between the two groups. A total of 50% (13/26) in BAF250a-retained
cases and 30% (3/10) in BAF250a-deficient cases (P=0.48) was
observed.
 | Table II.Tumor response of primary chemotherapy
in evaluable cases with ovarian clear cell adenocarcinoma. |
Table II.
Tumor response of primary chemotherapy
in evaluable cases with ovarian clear cell adenocarcinoma.
| RECIST
assessment | BAF250a-deficient
cases (n=10) | BAF250a-retained
cases (n=26) | P-value |
|---|
| Complete
response |
2
(20%) |
8
(31%) |
|
| Partial response |
1
(10%) |
5
(19%) |
|
| Stable disease |
1
(10%) |
3
(16%) |
|
| Progressive
disease |
6
(60%) |
10 (38%) |
|
| Response rate | 3/10 (30%) | 13/26 (50%) | 0.48 |
Overall and progression-free survival
of the patients
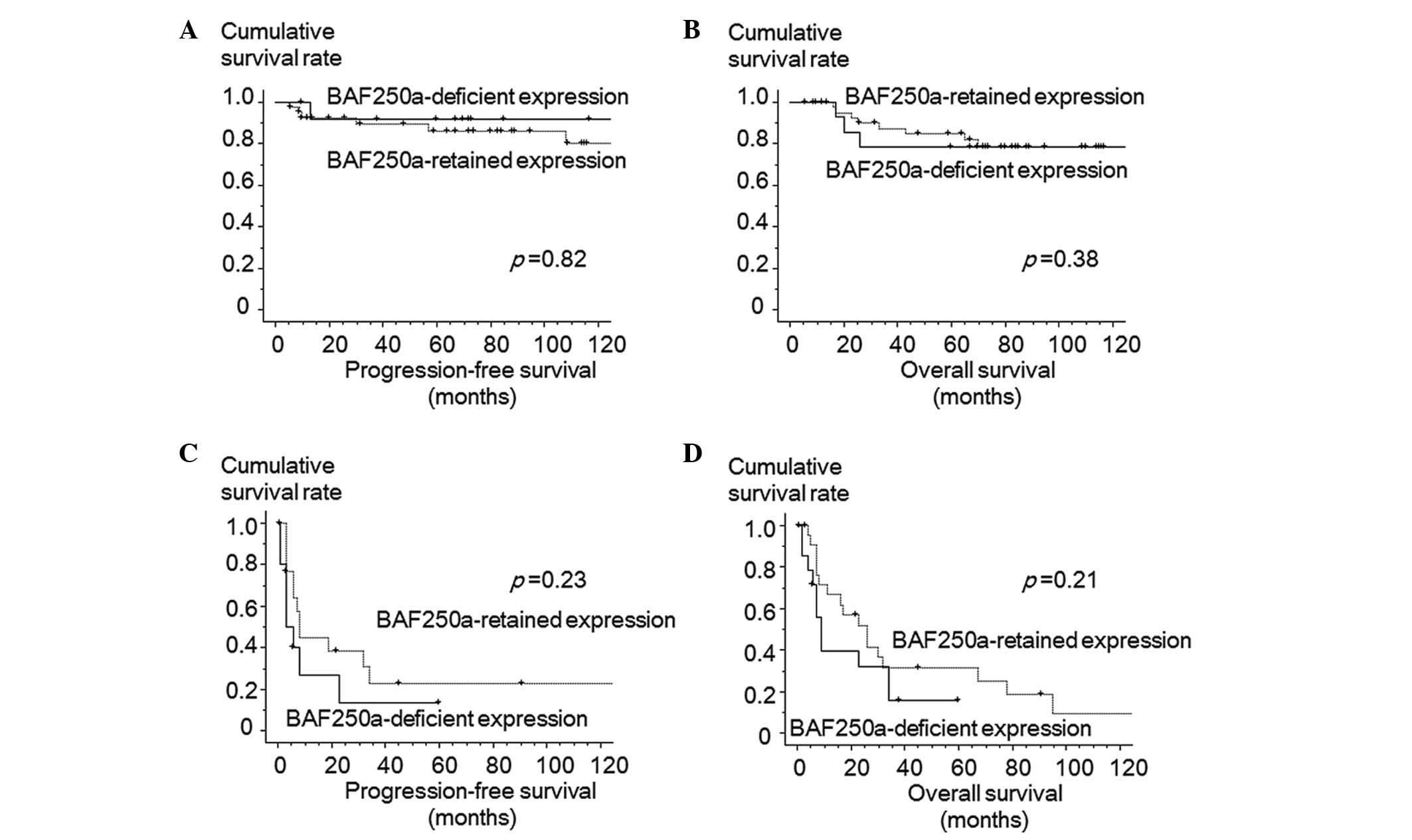
Kaplan-Meier survival curves of all patients are
shown in Fig. 2. BAF250a-deficient
expression status was not significantly correlated with
progression-free and overall survival of CCA in all enrolled cases.
The 5-year progression-free survival rates were 68% in
BAF250a-retained cases and 59% in BAF250a-deficient cases (P=0.24).
Additionally, the 5-year overall survival rates were 67% in
retained expression cases and 49% in deficient cases (P=0.43).
Kaplan-Meier survival curves of stage I/II patients
(Fig. 3A and B) and stage III/IV
patients (Fig. 3C and D), according
to BAF250a expression, were also shown. Additionally, no
significant differences were observed in the progression-free and
overall survival in patients with stage I/II cases. The 5-year
progression-free survival was 86% in BAF250a-retained cases and 91%
in BAF250a-deficient cases (P=0.82). The 5-year overall survival
rate was 85% in BAF250a-retained cases and 78% in BAF250a-deficient
cases (P=0.38). In cases with stage III/IV disease, no differences
were observed between the two groups. The 5-year progression-free
survival was 23% in BAF250a-retained cases and 13% in
BAF250a-deficient cases (P=0.23). The 5-year overall survival rate
was 25% in BAF250a-retained cases and 16% in BAF250a-deficient
cases (P=0.21).
BAF250a expression status is not an
independent prognostic factor
In the multivariate analysis for progression-free
survival, BAF250a expression status was not identified as an
independent prognostic factor (P=0.47; Table III). Residual tumor diameter was
identified as an independent factor for progression-free survival.
In the multivariate analysis for overall survival, age (P=0.01),
FIGO stage (P<0.01) and residual tumor diameter (P=0.02) were
prognostic factors; however, the BAF250a expression status was not
identified as an independent prognostic factor (P=0.56) in the
present cases.
 | Table III.Multivariate analyses for
progression-free survival and overall survival. |
Table III.
Multivariate analyses for
progression-free survival and overall survival.
|
| Progression-free
survival | Overall survival |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (continuous
variable) | 0.97 | 0.92–1.02 | 0.30 | 0.94 | 0.90–0.99 | 0.01 |
| Concurrence of
endometriosis (yes vs. no) | 0.78 | 0.32–1.86 | 0.57 | 0.96 | 0.47–1.94 | 0.90 |
| FIGO stage (III/IV
vs. I/II) | 9.10 | 3.33–25.0 | <0.01 | 6.17 | 2.60–14.6 | <0.01 |
| Residual tumor
(>1 cm vs. ≤1 cm) | 1.79 | 0.68–4.72 | 0.23 | 2.72 | 1.19–6.23 | 0.02 |
| BAF250a expression
(retained vs. deficient) | 0.72 | 0.32–1.74 | 0.47 | 0.80 | 0.39–1.67 | 0.56 |
Discussion
Frequencies of BAF250a-deficient expression in the
present study were 6% in solitary endometriosis, 19% in
non-atypical endometriosis, 26% in atypical endometriosis, 5% in
benign CCAF, 5% in borderline CCAF and 30% in CCA. Previous reports
have documented that frequencies of BAF250a-deficient expression in
solitary endometriosis, atypical endometriosis and CCA were 0–15,
38.5 and 15–66%, respectively (10,14,19–21).
When compared with solitary endometriosis and non-atypical
endometriosis synchronous with CCA, BAF250a-deficient expression
was more frequent in non-atypical endometriosis (6 vs. 19%),
suggesting this alteration was an early molecular alteration in the
development of CCA. In addition, BAF250a-deficient expression in
EM-related CCA and its precursor were more frequent compared with
that in CCAF-related CCA and its precursor. These findings
suggested that EM-related CCA may have different carcinogenic
pathway from CCAF-related CCA.
Systematic review of BAF250a in response to the
primary chemotherapy and prognosis in CCA was shown in Table IV. It remains controversial whether
BAF250a status is correlated with the chemoresistance of CCA. A
report demonstrated that loss of BAF250a expression was associated
with chemoresistance of CCA (14);
however, the others did not show any significant differences
(10,15–17). This
discrepancy may be simply explained by a sample size or different
patient characteristics. In the present study, BAF250a expression
status was not significantly correlated with response rate for
chemotherapy of CCA in accordance with the results of several
reports (10,15,16).
 | Table IV.Review of the impact of BAF250a on
the response to primary chemotherapy and prognoses in ovarian clear
cell adenocarcinoma. |
Table IV.
Review of the impact of BAF250a on
the response to primary chemotherapy and prognoses in ovarian clear
cell adenocarcinoma.
| Authors, year | No. of
patients | BAF250a-deficient
cases (%) | Response to primary
chemotherapy | Progression-free
survival | Overall
survival | Other factors
associated with BAF250a expression | Refs. |
|---|
| Yamamoto et
al, 2012 | 90 (Japanese) | 39 | BAF250a-deficient,
46% BAF250a-retained, 29% (P=0.25) | NE (P=0.12) | No association | Endometriosis | (10) |
| Katagiri et
al, 2012 | 60 (Japanese) | 15 | BAF250a-deficient,
0% BAF250a-retained, 60% (P=0.04) | Worse in
BAF250a-deficient cases (P<0.01) | No association
(P=0.15) | FIGO stage, CA125
regimen | (14) |
| Maeda et al,
2010 | 149 (89 Japanese;
60 Taiwanese) | 59 | NE | NE | No association
(P=0.97) | Macroscopic feature
(cystic vs. adenofibromatous) | (16) |
| Lowery et
al, 2012 | 82 (Canadian) | 41 | NE | NE | No association |
| (17) |
| Present study | 97 (Japanese) | 30 | BAF250a-deficient,
30% BAF250a-retained, 50% | No association
(P=0.47) | No association
(P=0.56) | Endometriosis,
CCAF | – |
In the present study, BAF250a-deficient cases
exhibited a relatively lower chemotherapy response and a worse
prognosis; however, BAF250a expression status was not significantly
associated with response rate or prognoses in CCA. Additionally,
multivariate analyses revealed that BAF250a status was not an
independent prognostic factor for progression-free survival and
overall survival. More important factors, including age, FIGO stage
and residual tumor diameter, were identified as prognostic factors
for overall survival in CCAs.
In conclusion, BAF250a expression status was not
identified as an independent prognostic factor for progression-free
survival or overall survival in patients with CCA. However,
BAF250a-deficient expression was closely correlated with early
tumorigenesis of endometriosis-related CCA. BAF250a expression was
closely associated with an early neoplastic process of
endometriosis and it may be a potential biomarker for detecting
early malignant transformation of endometriosis. Additionally, it
is necessary to investigate the method to detect the alteration of
BAF250a expression in the follow-up of the patients with
endometriosis. Further biomarker analyses, including BAF250a
expression, are required to improve the prognoses of CCAs.
References
|
1
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinomas have poorer prognosis
compared to other epithelial cell types? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itamochi H, Kigawa J and Terakawa N:
Mechanisms of chemoresistance and poor prognosis in ovarian clear
cell carcinoma. Cancer Sci. 99:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective muticentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gross AL, Kurman RJ, Vang R, Shih IeM and
Visvanathan K: Precursor lesions of high-grade serous ovarian
carcinoma: Morphological and molecular characteristics. J Oncol.
2010:1262952010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan DS and Kaye S: Ovarian clear cell
adenocarcinoma: A continuing enigma. J Clin Pathol. 60:355–360.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiegand KC, Shah SP, AlAgha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-assciated ovarian carcinoma.
N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones S, Wang TL, Shih IeM, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto S, Tsuda H, Takano M, Tamai S and
Matsubara O: PIK3CA mutaitons and loss of ARID1A protein expression
are early events in the development of cystic ovarian clear cell
adenocarcinoma. Virchows Arch. 460:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan DS, Iravani M, McCluggage WG, Lambros
MB, Milanezi F, Mackay A, Gourley C, Geyer FC, Vatcheva R, Millar
J, et al: Genomic analysis reveals the molecular heterogeneity of
ovarian clear cell carcinomas. Clin Cancer Res. 17:1521–1534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto S, Tsuda H, Yoshikawa T, Kudoh K,
Kita T, Furuya K, Tamai S and Matsubara O: Clear cell
adenocarcinoma associated with clear cell adenofibromatous
components: A subgroup of ovarian clear cell adenocarcinoma with
distinct clinicopathologic characteristics. Am J Surg Pathol.
31:999–1006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Veras E, Mao TL, Ayhan A, Ueda S, Lai H,
Hayran M, Shih IeM and Kurman RJ: Cystic and adenofibromatous clear
cell carcinomas of the ovary: Distinctive tumors that differ in
their pathogenesis and behavior: A clinicopathologic analysis of
122 cases. Am J Surg Pathol. 33:844–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katagiri A, Nakayama K, Rahman MT, Rahman
M, Katagiri H, Nakayama N, Ishikawa M, Ishibashi T, Iida K,
Kobayashi H, et al: Loss of ARID1A expression is related to shorter
progression-free survival and chemoresistance in ovarian clear cell
carcinoma. Mod Pathol. 25:282–288. 2012.PubMed/NCBI
|
|
15
|
Yokoyama Y, Matsushita Y, Shigeto T,
Futagami M and Mizunuma H: Decreased ARID1A is correlated with
chemoresistance in epithelial ovarian cancer. J Gynecol Oncol.
25:58–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda D, Mao TL, Fukuyamaa M, Nakagawa S,
Yano T, Taketani Y and Shih IeM: Clinicopathological significance
of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma.
Int J Mol Sci. 11:5120–5128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowery WJ, Schildkraut JM, Akushevich L,
Bentley R, Marks JR, Huntsman D and Berchuck A: Loss of
ARID1A-associated protein expression is a frequent event in clear
cell and endometrioid ovarian cancers. Int J Gynecol Cancer.
22:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato M, Yamamoto S, Takano M, Matsubara O
and Furuya K: Aberrant expression of mammalian target of rapamycin,
hypoxia-inducible factor-1α and glucose transporter 1 in the
development of ovarian clear-cell adenocarcinoma. Int J Gynecol
Pathol. 31:254–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samartzis EP, Samartzis N, Noske A, Fedier
A, Caduff R, Dedes KJ, Fink D and Imesch P: Loss of
ARID1A/BAF250a-expression in endometriosis: A biomarker for risk of
carcinogenic transformation? Mod Pathol. 25:885–892. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao W, Awadallah A and Xin W: Loss of
ARID1A/BAF250a expression in ovarian endometriosis and clear cell
carcinoma. Int J Clin Exp Pathol. 5:642–650. 2012.PubMed/NCBI
|
|
21
|
Ayhan A, Mao TL, Seckin T, Wu CH, Guan B,
Ogawa H, Futagami M, Mizukami H, Yokoyama Y, Kurman RJ and Shih
IeM: Loss of ARID1A expression is an early molecular event in tumor
progression from ovarian endometriotic cyst to clear cell and
endometrioid carcinoma. Int J Gynecol Cancer. 22:1310–1315. 2012.
View Article : Google Scholar : PubMed/NCBI
|