Introduction
It is estimated that over one-third of the world's
population has been infected with hepatitis B virus (HBV), and
patients who are seropositive for hepatitis B surface antigen
(HBsAg+) account for 12% of cancer patients receiving
chemotherapy (1,2). HBV reactivation during or after
chemotherapy and subsequent liver function impairment is a major
concern (3). HBV reactivation by
chemotherapy is widely reported in the literature for hematological
malignancies and lymphomas, and less for solid tumors (4). Lung cancer ranks first in incidence and
mortality worldwide (5); however, HBV
reactivation has been largely overlooked in this disease. Only one
study previously reported a reactivation rate of ~19% among
HBsAg+ patients with advanced non-small-cell lung cancer
(NSCLC) (6). However, as regards
small-cell lung cancer (SCLC), HBV reactivation has rarely been
reported.
We herein report the case of an HBsAg+
SCLC patient with HBV reactivation during the course of
chemotherapy, despite preemptive use of lamivudine. The patient
developed fulminant viral hepatitis and succumbed to subsequent
liver failure.
Case report
A 56-year-old man was admitted to the West China
Hospital (Chengdu, China) in September, 2014 due to cough and blood
in the sputum. The patient was diagnosed with SCLC of the right
lung (stage T3N2M0). In the diagnostic work-up, the serological
tests revealed positivity for HBsAg, HBeAg and HBcAb. The HBV DNA
load was 1.1×105 IU/ml. The patient had mildly elevated
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
levels [57 and 41 IU/l; upper limits of normal (ULN), 50 and 40
IU/l, respectively]. The biochemistry profile and complete blood
count were otherwise normal. The family history revealed that the
patient's mother and two brothers had died from liver disease.
The patient was prescribed preemptive lamivudine
(100 mg once daily) initiated 1 week prior to chemotherapy,
followed by combination chemotherapy (etoposide with carboplatin
for 3 cycles and with cisplatin for 1 cycle); he then received
radiotherapy to the thorax and mediastinum. Due to severe
hemoptysis, myelosuppression and acute pancreatitis, the treatment
was interrupted several times and was continued for a total of 6
months. During this time, the tumor enlarged, which could not be
readily attributed to chemotherapy failure. The patient later
received another 2 cycles of etoposide and cisplatin chemotherapy.
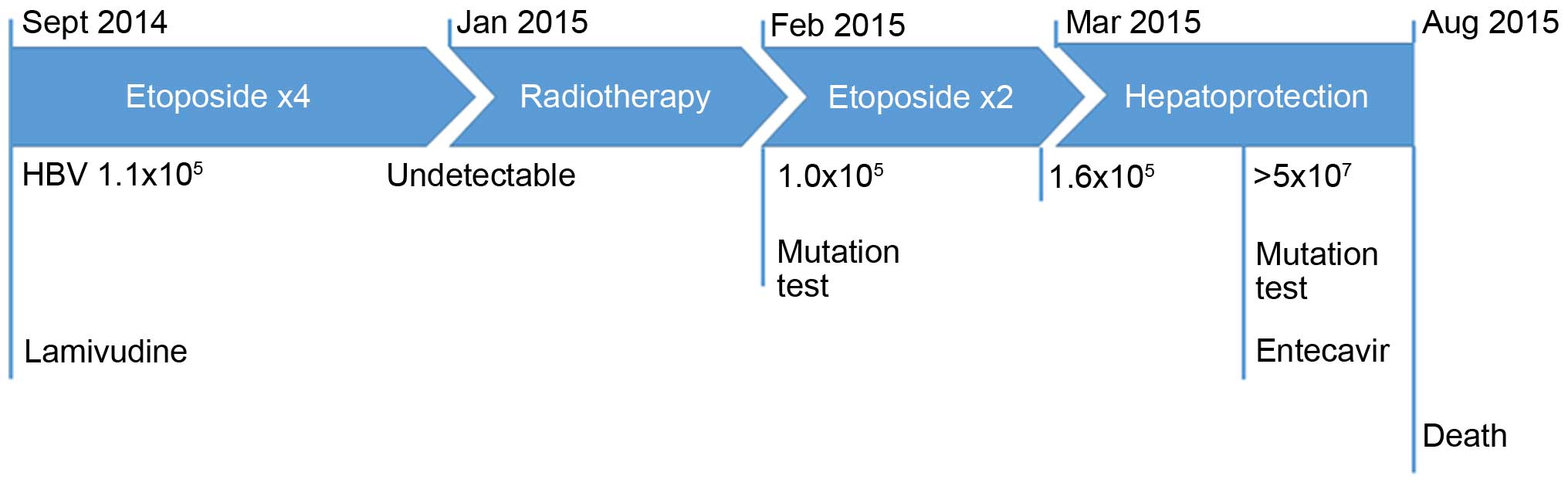
The treatment timeline is depicted in Fig. 1.
The HBV DNA load dropped below the detection limit
with the advent of lamivudine and remained low for >8 months.
However, it increased to 1.0×105 IU/ml after the
completion of thoracic radiotherapy. At this time point, an HBV
genetic analysis was performed (Table
I). Lamivudine resistance was not identified and the patient
continued with lamivudine administration. The HBV DNA decreased to
6.0×103 IU/ml and increased to 1.6×105 IU/ml
after the final 2 cycles of chemotherapy. The ALT and AST levels
remained stable (under the detection limit) for the entire
time.
 | Table I.The HBV genetic analysis. |
Table I.
The HBV genetic analysis.
| Mutation site | Abundance (test 1)
% | Abundance (test 2)
% |
|---|
| S213T | 50 | 100 |
| Q215H | 50 | 70 |
| M204I | 0 | 100 |
| V214A | 0 | 30 |
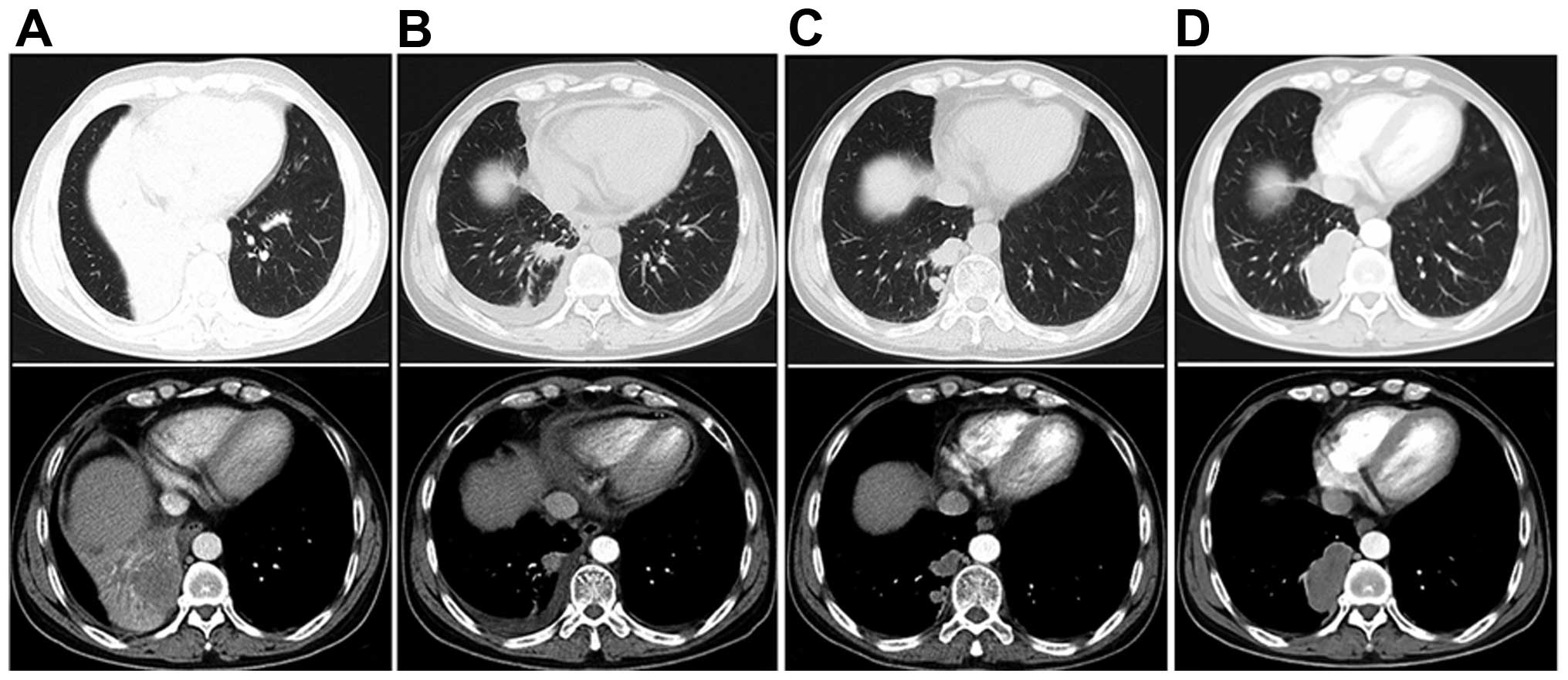
After 3 months, tumor progression was detected
(Fig. 2). The laboratory examination
revealed a sharply elevated ALT (from 117 to 557 IU/l), AST (from
184 to 886 IU/l), and total bilirubin levels [from 14.8 (ULN, 28.0
μmol/l) to 98.5 μmol/l], despite intensive use of hepatoprotectants
(Essentiale, Gluthion and Transmetil). The HBV DNA was
>5×107 IU/ml. The HBV mutation status was again
analyzed (Table I) and this time it
revealed a definitive emergence of lamivudine resistance. The
antiviral agent was switched to entecavir (0.5 mg once a day).
However, the patient developed fulminant hepatitis and his
condition progressively deteriorated, with emerging signs of
cholenzyme separation, hemostatic dysfunction, hypoalbuminemia,
hyperammonemia, hepatic encephalopathy and hepatorenal syndrome.
Eventually, the patient succumbed to the disease on August 25,
2015. Autopsy was refused.
Discussion
We herein report the case of a SCLC patient who
developed HBV reactivation during chemotherapy, despite preemptive
lamivudine treatment. The HBV DNA level rebounded after the initial
drop, together with the appearance of lamivudine resistance
mutations in the viral gene. In addition, the disease progressed
under continuous lamivudine administration. This was accompanied by
significant deterioration of hepatic function, and the patient
eventually succumbed to the disease.
The definition of HBV reactivation varies in the
literature, but it commonly refers to an ALT level >2 ULN in
combination with either an abrupt increase in HBV replication of 1
log10 or an absolute value >2×104 IU/ml
(7). In the majority of the cases,
HBV reactivation occurred 1 week-3 months post-chemotherapy
(8). The patient's HBV DNA increased
to 1.0×105 IU/ml 3 months after the fourth cycle of
chemotherapy. The time course was typical of chemotherapy-induced
HBV reactivation. Furthermore, the virus genetic analysis revealed
lamivudine resistance mutations. Considering the full course of HBV
reactivation in a background of chemotherapy, it was reasonable to
hypothesize that the chemotherapy was responsible for the HBV
reactivation.
Chemotherapy-induced HBV reactivation has been
extensively investigated. In retrospective series, the rate of HBV
reactivation was as high as 80% in lymphomas or other hematological
malignancies, particularly those treated with immunoinhibitory
rituximab (3). The incidence of HBV
reactivation in lung cancer had not been elucidated until recently,
when a retrospective study reported a rate of 19% in NSCLC patients
(6). However, SCLC patients have not
been investigated, with no studies specific to SCLC patients
reported to date. To the best of our knowledge, this is the only
reported case of chemotherapy-induced fatal HBV reactivation in a
SCLC patient.
The reasons underlying the lack of reports on HBV
reactivation in SCLC patients remains largely unknown, although it
may be due to the low incidence of this complication. Patients with
hematological malignancies and lymphomas have the highest rates of
HBV reactivation, mainly due to the intense chemotherapy and
administration of immunoinhibitory monoclonal antibodies; in
addition, such patients are already immunocompromised (3). Breast cancer patients also exhibited
high rates of HBV reactivation, mainly attributed to the wide use
of anthracyclines and corticosteroids. The HBV reactivation rates
were lower in other cancer types. Another possibility is that SCLC
has a dismal prognosis, and patients may become seriously ill
before HBV reactivation is detected.
The reason underlying the poor prognosis is the
evolution of the HBV gene, as evidenced by the serial monitoring of
the lamivudine resistance gene. However, the patient's family
history of liver disease must be taken into consideration, and the
possibility of liver fragility cannot be ruled out. In addition,
the patient had other comorbidities, such as severe hemoptysis,
myelosuppression and acute pancreatitis. Thus, the fatal outcome
may be attributed to a combination of all these factors.
Preemptive antiviral therapy is recommended for
HBsAg+ cancer patients prior to the initiation of
chemotherapy (3,6,9).
Accumulating data demonstrated that lamivudine reduced the
incidence of HBV reactivation and the severity of hepatitis in
these patients (10–13). The value of novel antiviral drugs,
such as entecavir, telbuvidine, adefovir and tenofovir, is being
evaluated. Recently, a trial compared the efficacy of lamivudine
and entecavir in HBsAg+ patients with diffuse large
B-cell lymphoma undergoing rituximab treatment (14). Compared with lamivudine, entecavir
significantly reduced the incidence of HBV reactivation and
HBV-related hepatitis. It was reasonable to hypothesize that
high-risk patients may benefit from the usage of entecavir rather
than lamivudine.
In summary, this was a rare case of SCLC with fatal
HBV reactivation during the chemotherapy course. This aim of this
report was to highlight the major issue of HBV reactivation in
SCLC, which is frequently overlooked, and suggest that agents more
potent than lamivudine may be more efficacious for high-risk
patients.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81272684).
References
|
1
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeo W, Chan PK, Zhong S, Ho WM, Steinberg
JL, Tam JS, Hui P, Leung NW, Zee B and Johnson PJ: Frequency of
hepatitis B virus reactivation in cancer patients undergoing
cytotoxic chemotherapy: A prospective study of 626 patients with
identification of risk factors. J Med Virol. 62:299–307. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mandala M, Fagiuoli S, Francisci D, Bruno
R, Merelli B, Pasulo L, Tondini C, Labianca R and Roila F:
Hepatitis B in immunosuppressed cancer patients: Pathogenesis,
incidence and prophylaxis. Crit Rev Oncol Hematol. 87:12–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeo W and Johnson PJ: Diagnosis,
prevention and management of hepatitis B virus reactivation during
anticancer therapy. Hepatology. 43:209–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin GN, Peng JW, Xiao JJ, Liu DY and Xia
ZJ: Hepatitis B virus reactivation in hepatitis B surface antigen
seropositive patients with metastatic non-small cell lung cancer
receiving cytotoxic chemotherapy: The efficacy of preemptive
lamivudine and identification of risk factors. Med Oncol.
31:1192014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liaw YF and Chu CM: Hepatitis B virus
infection. Lancet. 373:582–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoofnagle JH, Doo E, Liang TJ, Fleischer R
and Lok AS: Management of hepatitis B: Summary of a clinical
research workshop. Hepatology. 45:1056–1075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lok AS and McMahon BJ: Chronic hepatitis
B: Update 2009. Hepatology. 50:661–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei
KI, Chan AT, Mok TS, Lee JJ, Leung TW, et al: Lamivudine for the
prevention of hepatitis B virus reactivation in hepatitis B
s-antigen seropositive cancer patients undergoing cytotoxic
chemotherapy. J Clin Oncol. 22:927–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY,
Lai LS, Cheung M, Zhang HY, Lie A, Ngan R and Liang R: Early is
superior to deferred preemptive lamivudine therapy for hepatitis B
patients undergoing chemotherapy. Gastroenterology. 125:1742–1749.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang
MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC, et al: A revisit
of prophylactic lamivudine for chemotherapy-associated hepatitis B
reactivation in non-Hodgkin's lymphoma: A randomized trial.
Hepatology. 47:844–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long M, Jia W, Li S, Jin L, Wu J, Rao N,
Feng H, Chen K, Deng H, Liu F, et al: A single-center, prospective
and randomized controlled study: Can the prophylactic use of
lamivudine prevent hepatitis B virus reactivation in hepatitis B
s-antigen seropositive breast cancer patients during chemotherapy?
Breast Cancer Res Treat. 127:705–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang H, Li X, Zhu J, Ye S, Zhang H, Wang
W, Wu X, Peng J, Xu B, Lin Y, et al: Entecavir vs lamivudine for
prevention of hepatitis B virus reactivation among patients with
untreated diffuse large B-cell lymphoma receiving R-CHOP
chemotherapy: A randomized clinical trial. JAMA. 312:2521–2530.
2014. View Article : Google Scholar : PubMed/NCBI
|