Introduction
Palliative combination chemotherapy has become the
standard of care for patients with advanced gastric cancer, as it
is effective in prolonging survival and improving the quality of
life (1). The short-term efficacy is
usually assessed after 2 or 3 cycles of chemotherapy. The objective
response (OR) to chemotherapy is the primary study endpoint, which
is essential for assessing prognosis and planning further treatment
(2). However, ~50% of patients with
advanced gastric cancer may not benefit from chemotherapy, which
may be determined prior to the second cycle of chemotherapy.
Computed tomography (CT) scans are often used by physicians to
evaluate the OR to chemotherapy in cancer patients (3). However, due to its radiation-associated
risks and low sensitivity, CT is not suitable for assessing the OR
to chemotherapy within a shorter period of time.
Identifying these non-responding patients within a
shorter time period may represent a challenge for most physicians.
Thus, the development of easier, cost-effective and safer tools for
monitoring the effects of chemotherapy in gastric cancer patients
would be of great value. Several studies recently investigated the
prognostic relevance of circulating tumor cell (CTC) count in
patients with solid tumors (4,5),
including gastric cancer (6).
Hematogenous metastasis is one of the main ways of malignant tumor
metastasis. Thus, the majority of patients with advanced cancer
have tumor cells in their peripheral blood. Detection of CTCs may
be more sensitive compared with CT and other imaging tests for
monitoring the chemotherapeutic effects. In this study,
immunomagnetic enrichment was used to isolate and purify CTCs from
peripheral blood, followed by fluorescein isothiocyanate
(FITC)-labeled anti-cytokeratin (CK) 7/8/18/19 antibody staining
and fluorescence microscope identification of CTCs, in order to
investigate the prognostic and predictive values of CTC count
determination in advanced gastric cancer patients who receive
palliative combination chemotherapy.
Materials and methods
Patients and treatment protocols
Tumor specimens were collected from 59 gastric
cancer patients with stage II–IV disease, who were recruited
between January, 2011 and June, 2013 and underwent chemotherapy at
the Department of Oncology, Changzhou Tumor Hospital (Changzhou,
China). The patients comprised 35 men and 24 women, with a median
age of 59 years (range, 35–81 years). None of the patients had
received previous chemotherapy. Histologically or cytologically
confirmed gastric cancer and confirmation of the clinical stage
based on the results of examination by chest X-ray and CT scan of
the chest and abdomen. All the patients received at least 3 cycles
of paclitaxel at 60 mg/m2 i.v. guttae on days 1, 8 and
15, with cisplatin 25 mg/m2 i.v. guttae on days 1–3,
followed by continuous infusion of 500 mg/m2
5-fluorouracil on days 1–5.
This study was approved by the local Ethics
Committee and written informed consent was obtained from all the
patients prior to enrolment.
Remission analysis
Tumor response was assessed according to the
Response Evaluation Criteria in Solid Tumors (7): Complete response (CR) was defined as
disappearance of all target lesions; partial response (PR) was
defined as at least a 30% decrease in the sum of the diameters of
target lesions, taking as reference the baseline sum of the
diameters; progressive disease (PD) was defined as at least a 20%
increase in the sum of the diameters of the target lesions, taking
as reference the smallest sum on study. In addition to the relative
increase of 20%, the sum must also demonstrate an absolute increase
of ≤5 mm; and stable disease (SD) was defined as neither sufficient
shrinkage to qualify as PR nor sufficient increase to qualify as
PD, taking as reference the smallest sum of the diameters while on
study.
Follow-up
Interim history, physical examination, hematological
studies, measurement of serum carcinoembryonic antigen (CEA) and
carbohydrate antigen (CA) 19-9 levels and whole-body CT were
performed every 3 months during the first year and every 6 months
thereafter. Progression-free survival (PFS) was defined as the time
from study entry until disease progression, death, or the day of
the last follow-up visit, whichever came first. Overall survival
(OS) was defined as the time from study entry until the date of
death, regardless of the cause, or the most recent documented
follow-up visit.
Blood sample collection from patients
and healthy donors
Peripheral blood samples were collected to
investigate the presence of CTCs prior to the administration of the
first cycle of chemotherapy (baseline) and prior to the second
cycle of chemotherapy in patients with advanced gastric cancer. For
blood spiking experiments and to be used as controls, peripheral
blood samples were also collected from 9 healthy donors. All the
blood samples were collected in EDTA-coated tubes
(S-Monovette®; Sarstedt, Nümbrecht, Germany). To avoid
epithelial cell contamination from the skin puncture, the first 5
ml of peripheral blood were stored for other studies. After
collection, the blood samples were immediately processed for
further experiments.
Cell spiking experiments
For cell spiking experiments, SGC-7901 gastric
cancer cells expressing epithelial cell adhesion molecule (EpCAM),
CK7, CK8, CK18 and CK19, were serially diluted in 5-ml blood
samples collected from 5 different healthy donors. The dilutions
applied were as follows: 103, 102, 10 and 0
SGC-7901 cells per 5 ml whole blood. The SGC-7901 human gastric
cancer cell line was obtained from the Shanghai Cell Bank of
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA),
supplemented with 10% bovine serum, penicillin (100 U
ml−1), streptomycin (100 µg ml−1), pyruvate,
glutamine and insulin at 37°C in a water-saturated atmosphere with
5% CO2. The collection of cells was performed using
Trypsin-EDTA (Gibco BRL) and centrifugation at room temperature for
3 min at 300 × g. The cells were then counted using a hemocytometer
(Ningbo Hinotek Co., Ltd., Zhejiang, China) and cell viability was
confirmed by trypan blue staining.
Mononuclear cell (MNC) collection
The MNC population was extracted according to the
following protocol: 5-ml peripheral blood samples were carefully
layered onto a 15-ml Ficoll gradient (FicoLite-H®,
density: 1.077 g/ml; Linaris, Wertheim, Germany) and covered with
10 ml phosphate-buffered saline (PBS) solution (PAA Laboratories
GmbH, Pasching, Austria). The samples were spun in a centrifuge at
4°C for 30 min at 300 × g without brake. Concentrated MNCs were
harvested from the interface using a disposable pipette. The
isolated cells were washed once in PBS, spun in a centrifuge for 10
min at 300 × g and resuspended in 1 ml PBS. The MNCs were counted
with a hemocytometer and resuspended at a density of 107
cells/ml in PBS.
Immunomagnetic enrichment and
immunofluorescence staining
The blood samples from the patients and the spiked
(with SGC-7901 cells) blood samples (5 ml blood for each
experiment) of healthy volunteers were processed using CTC
immunoisolation with the CELLection™ Epithelial Enrich kit
following manufacturer's protocol (Invitrogen Dynal, Oslo, Norway).
Following EpCAM-based immune enrichment, the isolated CTCs were
stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI)
(Roche Molecular Biochemicals, Mannheim, Germany) and FITC-labeled
anti-CK7/8/18/19 antibody (polyclonal, rabbit, specific for
CK7/8/18/19 of human; dilution, 1:200, cat. no. RE-1588R-FITC;
Yanjing Biotech Co., Ltd., Shanghai, China) following the
manufacturer's protocol; subsequently, the CTCs were counted by
fluorescence microscopy. CTCs were identified as the cells showing
a fluorescent signal of anti-CK7/8/18/19-FITC in the cytoplasm and
specific DAPI staining in the nucleus. Finally, in cell spiking
experiments, we calculated the percentage of CTC recovery as
follows: CTC recovery % = no. of CTCs recovered from 5 ml blood/no.
of SGC-7901 cells added to 5 ml blood × 100%.
Statistical methods
Statistical significance was based on a two-sided
significance level of 0.05. All the analyses were performed with
SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). The
differences in the values among the groups under study were
assessed using the analysis of independent samples t-test, as
indicated. The association between CTC count and
clinicopathological characteristics was analyzed by the Chi-square
test. The paired-samples t-test was used to compare CTC counts
prior to and following chemotherapy. Kaplan-Meier survival curves
and the log-rank test were used to analyze univariate distributions
for PFS and OS. The prognostic significance of baseline CTC count
and changes of the CTC count following chemotherapy was assessed
using Cox proportional hazards regression analysis. P≤0.05 was
considered to indicate statistically significant differences.
Results
Specificity and sensitivity of
enrichment and extraction protocols
No CK signal was observed in the examined blood
samples from the 9 healthy donors, which demonstrated the
specificity of the used assays. In serial dilution assays, 10
SGC-7901 cells were detected in 5 ml whole blood, which was
repeated in 5 healthy donors. Finally, the percentage of CTC
recovery was calculated (Table
I).
 | Table I.Circulating tumor cell (CTC) recovery
percentages in cell spiking experiments. |
Table I.
Circulating tumor cell (CTC) recovery
percentages in cell spiking experiments.
|
| CTC number (recovery
percentage %) |
|---|
|
|
|
|---|
| SGC7901 cell
number | A | B | C | D | E | Mean value ± SD |
|---|
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0±0 (0.0±0.0) |
| 10 | 5 (50.0) | 4 (40.0) | 1 (10.0) | 3 (30.0) | 6 (60.0) | 3.8±1.9
(38.0±19.2) |
| 100 | 39 (39.0) | 43 (43.0) | 37 (37.0) | 52 (52.0) | 41 (41.0) | 42.4±5.9
(42.4±5.9) |
| 1,000 | 361 (36.1) | 432 (43.2) | 422 (42.2) | 415 (41.5) | 523 (52.3) | 430.6±58.5
(43.06±5.9) |
CTC counts in patients and healthy
controls
The average CTC count ± standard deviation in all 59
patients was 5.95±8.4/5 ml blood; no CK signal was detected in the
blood samples from the 9 healthy controls (0/5 ml blood). The
CTC-positive rate in the advanced gastric cancer and healthy
control groups was 83.05 and 0%, respectively. In 29 patients who
were tested twice, before and after the first cycle of
chemotherapy, the mean CTC count (8.10±12.64/5 ml blood) did not
decrease compared with that prior to chemotherapy (7.24±10.942/5 ml
blood) (P=0.527).
Associations between CTC count and
clinicopathological characteristics
High CTC counts were significantly associated with
poor tumor differentiation (P=0.021) and high serum CEA levels
(P=0.005), but not with age, gender, clinical stage or performance
status (Table II). It appears that
higher serum CA19-9 levels were associated with higher CTC counts,
but the difference was not statistically significant (P=0.078)
(Table II).
 | Table II.Associations between CTC counts and
clinicopathological features. |
Table II.
Associations between CTC counts and
clinicopathological features.
|
| CTC |
|
|
|---|
|
|
|
|
|
|---|
| Type | ≤ 2/5 ml blood | > 2/5 ml
blood | χ2 | P-value |
|---|
| Age (median, 59
years) |
|
| 2.07 | 0.121 |
| ≤59 | 9 | 21 |
|
|
|
>59 | 14 | 15 |
|
|
| Gender |
|
| 3.326 | 0.069 |
|
Male | 17 | 18 |
|
|
|
Female | 6 | 18 |
|
|
| CEA, ng/ml |
|
| 8.361 | 0.005 |
|
<5 | 20 | 18 |
|
|
| ≥5 | 3 | 18 |
|
|
| CA19-9 ng/ml |
|
| 2.842 | 0.078 |
|
<37 | 16 | 17 |
|
|
|
≥37 | 7 | 19 |
|
|
| Stage |
|
| 0.116 | 0.762 |
|
III | 6 | 8 |
|
|
| IV | 17 | 28 |
|
|
| ECOG PS |
|
| 0.116 | 0.762 |
|
0,1 | 6 | 8 |
|
|
| 2 | 17 | 28 |
|
|
| Tumor
differentiation |
|
| 5.281 | 0.021 |
|
Poor/undifferentiated | 9 | 25 |
|
|
| High
and moderate | 14 | 11 |
|
|
Associations between changes in CTC
count and response in patients tested before and after the first
cycle of chemotherapy
Changes in the CTC count in 29 patients who were
tested before and after the first cycle of chemotherapy were
significantly correlated with efficacy after 3 cycles of
chemotherapy. The mean CTC count (1.2±2.04 cells/5 ml blood) after
the first cycle of chemotherapy was significantly decreased in
patients who obtained CR and PR (n=15), compared with that prior to
chemotherapy (2.53±3.75 cells/5 ml blood) (P=0.049). In patients
with PD (n=7), the mean CTC count (23.43±17.01/5 ml blood)
increased significantly, compared with that prior to chemotherapy
(14.71±16.71/5 ml blood) (P=0.021). The CTC count after 1 month of
chemotherapy did not decrease significantly in patients with SD
compared with that prior to chemotherapy.
Associations between CTC count and
survival
For all patients, the median PFS was 5 months
(range, 2–43 months), and the median OS was 11 months (range, 4–45
months). In all patients, CTC count was significantly associated
with PFS (P<0.001) and OS (P<0.001) (Table III). Other factors that were
significantly associated with PFS and OS on univariable analysis
using Kaplan-Meier survival curves and the log-rank test were
Eastern Cooperative Oncology Group performance status, tumor
response and the serum levels of CEA and CA19-9 (Table III). The Kaplan-Meier survival
curve for patients with CTC counts ≤ and >2 cells/5 ml
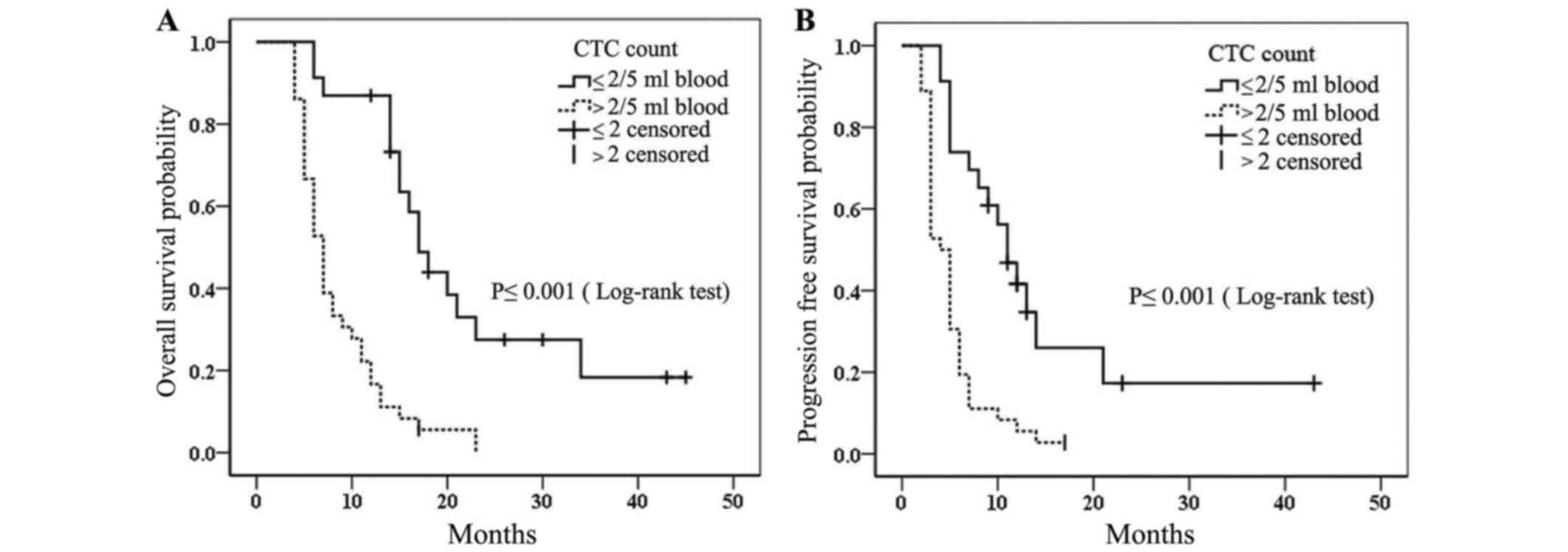
peripheral blood are shown in Fig.
1. Patients with CTC counts ≤2 cells/5 ml peripheral blood had
a significantly longer median PFS and median OS compared with
patients with CTC counts >2 cells/5 ml peripheral blood (median
PFS, 11 vs. 4 months; and median OS, 17 vs. 7 months,
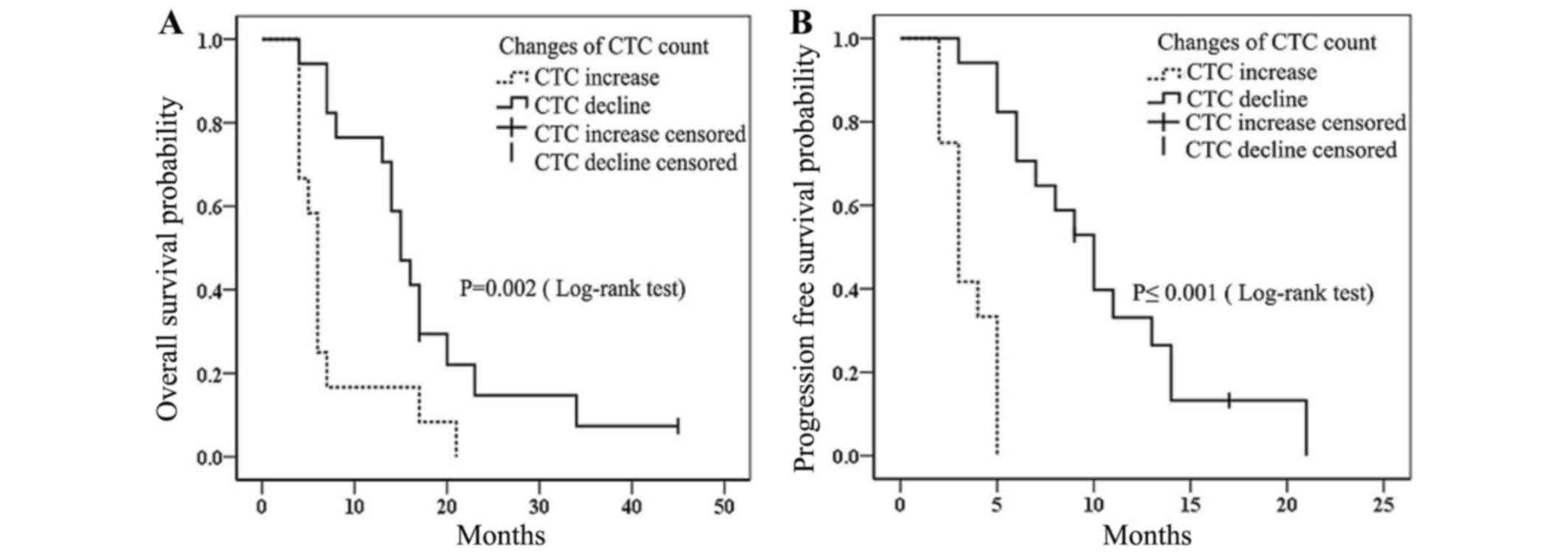
respectively). In the 29 patients tested before and after the first
cycle of chemotherapy, we found that patients exhibiting CTC count
decline had significantly longer median PFS and OS compared with
those with CTC count increase (median PFS, 10 vs. 3 months,
respectively; P≤0.001; and median OS, 15 vs. 6 months,
respectively; P=0.002). (Table
III; Fig. 2).
 | Table III.Factors associated with survival in
59 patients receiving chemotherapy. |
Table III.
Factors associated with survival in
59 patients receiving chemotherapy.
| Prognostic
factors | n | Median PFS
(months) | P-value | MST (months) | P-value |
|---|
| Gender |
|
| 0.32 |
| 0.208 |
|
Male | 35 | 6 |
| 11 |
|
|
Female | 24 | 5 |
| 7 |
|
| Age, years |
|
| 0.221 |
| 0.169 |
|
≤59 | 30 | 5 |
| 7 |
|
|
>59 | 29 | 6 |
| 14 |
|
| Tumor
differentiation |
|
| 0.141 |
| 0.072 |
|
Poor/undifferentiated | 34 | 5 |
| 7 |
|
| High
and moderate | 25 | 7 |
| 14 |
|
| ECOG PS |
|
| 0.002 |
| 0.001 |
|
0,1 | 36 | 7 |
| 15 |
|
| 0 | 23 | 4 |
| 6 |
|
| Response |
|
| 0.01 |
| 0.002 |
|
CR+PR | 29 | 11 |
| 17 |
|
|
SD+PD | 30 | 3 |
| 6 |
|
| CA19-9 ng/ml |
|
| 0.01 |
| 0.002 |
|
≤37 | 33 | 7 |
| 14 |
|
|
>37 | 26 | 4 |
| 6 |
|
| Stage |
|
| 0.760 |
| 0.800 |
|
III | 14 | 5 |
| 7 |
|
| IV | 45 | 5 |
| 12 |
|
| CTC |
|
| <0.001 |
| <0.001 |
| ≤2
cells/5 ml peripheral blood | 23 | 11 |
| 17 |
|
| >2
cells/5 ml peripheral blood | 36 | 4 |
| 7 |
|
| Changes in CTC |
|
| <0.001 |
| <0.001 |
|
Decline | 17 | 10 |
| 15 |
|
|
Increase | 12 | 3 |
| 6 |
|
| CEA ng/ml |
|
| <0.001 |
| <0.001 |
| ≤5 | 38 | 7 |
| 14 |
|
|
>5 | 21 | 3 |
| 6 |
|
The multivariate analysis suggested that a CTC count
at baseline of >2 cells/5 ml blood was an independent poor
prognostic marker for PFS (hazard ratio = 2.81, 95% confidence
interval: 1.313–5.999, P=0.008) and OS (hazard ratio = 3.59, 95%
confidence interval: 1.655–7.817, P=0.001) in all 59 patients
(Table IV). However, in the 29
patients who were tested before and after the first cycle of
chemotherapy, CTC count increase was an independent poor prognostic
marker only for PFS (hazard ratio = 6.58, 95% confidence interval:
1.37–31.6, P=0.019) (Table IV).
 | Table IV.Hazard ratios for progression-free
and overall survival. |
Table IV.
Hazard ratios for progression-free
and overall survival.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Prognostic
factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Patients tested for
CTCs only prior to chemotherapy |
|
|
|
|
|
|
| CTC
count |
|
| 0.008 |
|
| 0.001 |
|
≤2 cells/5 ml
blood | 1 |
|
| 1 |
|
|
|
>2 cells/5 ml
blood | 2.81 | 1.313–5.999 |
| 3.59 | 1.655–7.817 |
|
| CA19-9
ng/ml |
|
| 0.039 |
|
| 0.007 |
|
≤37 | 1 |
|
| 1 |
|
|
|
>37 | 1.912 | 1.033–3.537 |
| 2.613 | 1.307–5.222 |
|
| CEA
ng/ml |
|
| 0.001 |
|
| 0.001 |
|
≤5 | 1 |
|
| 1 |
|
|
|
>5 | 3.460 | 1.685–7.107 |
| 3.672 | 1.781–7.571 |
|
| Patients tested for
CTCs before and after the first cycle of chemotherapy |
|
|
|
|
|
|
| Changes
in CTC |
|
| 0.019 |
|
| 0.638 |
|
Decline | 1 |
|
| 1 |
|
|
|
Increase | 6.58 | 1.37–31.6 |
| 0.774 | 0.266–2.251 |
|
| CA19-9
ng/ml |
|
| 0.708 |
|
| 0.003 |
|
≤37 | 1 |
|
| 1 |
|
|
|
>37 | 0.851 | 0.366–1.977 |
| 0.182 | 0.059–0.563 |
|
| CEA
ng/ml |
|
| 0.001 |
|
| 0.395 |
|
≤5 | 1 |
|
| 1 |
|
|
|
>5 | 0.173 | 0.061–0.489 |
| 0.566 | 0.152–2.103 |
|
Discussion
We demonstrated that a combination of immunomagnetic
separation of CTCs followed by FITC-labeled anti-CK7/8/18/19
antibody staining and fluorescence microscope identification of
CTCs may serve as a prognostic tool for PFS and OS in patients with
advanced gastric cancer receiving chemotherapy. In particular,
changes in the CTC count after the first cycle of chemotherapy may
serve as a prognostic tool for PFS and may predict the sensitivity
to chemotherapy regimens.
Solid tumor cells may enter the circulation, spread
to other tissues and initiate metastasis. Thus, tumor cells may be
detected in the peripheral blood. A variety of techniques and
instruments were recently developed to enrich and isolate CTCs from
the peripheral blood. These techniques generally rely on cell
surface antigen expression for capturing CTCs or cell size to
enrichment of CTCs by filtration techniques. CTC isolation methods
and instruments were elaborately reported recently (8–11). In
our study, we used commercially available immunomagnetic beads
coated with the monoclonal antibody Ber-EP4 (12), which recognizes specific epitopes of
the extracellular domain of the EpCAM molecule. EpCAM is expressed
only in epithelium and malignant tumors derived from epithelia;
thus, it may be used to enrich and isolate CTCs from blood. CKs are
major structural proteins of epithelial cells and comprise at least
20 members. These CKs are primarily expressed in normal epithelial
tissues, such as lung, gastrointestinal tract and kidney, as well
as in cancer cells arising from these tissues (13–17). We
adopted DAPI/FITC-labeled anti-CK7/8/18/19 antibody double staining
to identify CTCs with fluorescence microscopy. No CK signal was
observed in the blood samples from healthy donors, which verified
the specificity of the used assays. In serial dilution assays, 10
SGC-7901 cells were detected in 5 ml whole blood from 5 independent
healthy donors. Most of the CTC recovery percentages were >40%.
Low CTC recovery percentages may be correlated with the short
half-life of CTCs in the blood (18)
and relative long time of experimental procedure, although the
viable tumor cells were counted prior to the tumor cell spiking
experiments. In all the patients, The CTC-positive rate was 83.05%.
The high CTC-positive rate may be attributed to the fact that most
of the patients in our study had stage IV disease. High CTC counts
were also associated with poor tumor differentiation and high serum
CEA levels. We hypothesized that poorly differentiated tumor cells
exhibit strong invasiveness, and are therefore more likely to be
transferred to the blood and distant organs. Our results are
consistent with the available related literature (19,20).
CTCs may be detected in the peripheral blood of
patients with various cancers (21,22);
therefore, they may be used as an important auxiliary marker for
the diagnosis of malignant solid tumors (23,24).
However, the majority of gastric cancer patients have middle- or
late-stage disease at diagnosis. Hematogeneous tumor cell
dissemination is a key step in cancer progression. Therefore,
compared with its diagnostic value, CTC count may be more valuable
in predicting the sensitivity to chemotherapeutic agents or the
prognosis in patients with advanced gastric cancer. Several studies
have reported that CTCs may be used as prognostic or predictive
markers in patients with solid tumors, including gastric cancer
(20,25,26). In
our study, we observed that patients with low CTC counts (≤2
cells/5 ml peripheral blood) had a significantly longer median PFS
and median OS compared with patients with high CTC counts (>2
cells/5 ml peripheral blood). A high CTC count may also be an
independent poor prognostic marker for PFS and OS.
In this study, we also investigated whether the
changes in the CTC count were predictive of response to treatment,
although only 29 patients were tested for CTCs before and after the
first cycle of chemotherapy. In clinical practice, we often assess
the OR to chemotherapy after the 3rd cycle of chemotherapy by CT
scan or other imaging modalities. In fact, ~30% of the patients are
not likely to benefit from chemotherapy regimens. We consider that
CTC detection may be more sensitive compared with CT and other
imaging techniques in monitoring chemotherapeutic efficacy. Our
results from small samples demonstrated that the mean CTC counts
decreased significantly after the first cycle of chemotherapy in
patients who obtained CR and PR, compared with those prior to
chemotherapy (P=0.049). By contrast, in patients with PD, the mean
CTC counts after the first cycle of chemotherapy increased
significantly (P=0.021). We also found that patients with CTC count
decline had significantly longer median PFS and OS compared with
those with CTC count increase (Table
III; Fig. 2A and B). In 29
patients who were tested for CTCs before and after the first cycle
of chemotherapy, multivariate analysis suggested that CTC count
increase is an independent poor prognostic marker only for PFS
(hazard ratio = 6.58, 95% confidence interval: 1.37–31.6, P=0.019)
(Table IV). Our results may be
partly consistent with the reports from Matsushita et al and
other researchers (5,27–30). On
the contrary, other studies considered changes in CTC count during
the course of chemotherapy to not be predictive of clinical outcome
or response to therapy (31,32). The conflicting results between
different studies may be associated with variations in the
chemotherapeutic protocol or the different techniques used for
detecting CTCs.
One of limitations of the present study is the
relatively small size of the sample, which may explain the weak
prognostic value of the changes in CTC count for OS in the 29
patients tested before and after the first cycle of chemotherapy.
To further confirm the prognostic value of CTC testing in advanced
gastric cancer patients, future large-sample, multicenter
prospective studies are required.
Acknowledgements
The present study was partly supported by the
Science and Technology Planning Project of Changzhou, Jiangsu
Province (grant nos. CE20135051 and CE20165052), the Science and
Technology Planning Project of Changzhou Health Bureau (grant nos.
ZD201203 and ZD201616), the Research Project of the Health
Department of Jiangsu Province (grant nos. Z201221 and Z201616),
the 333 Talents Training Project of Jiangsu Province, the Key
Medical Innovation Talents Training Project of Changzhou (grant no.
2016CZLJ021), and the Project of Jiangsu Province Sanitation
Innovation Team (grant no. LJ201157).
References
|
1
|
Lordick F, Lorenzen S, Yamada Y and Ilson
D: Optimal chemotherapy for advanced gastric cancer: Is there a
global consensus? Gastric Cancer. 17:213–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Methy N, Bedenne L and Bonnetain F:
Surrogate endpoints for overall survival in digestive oncology
trials: Which candidates? A questionnaires survey among clinicians
and methodologists. BMC cancer. 10:2772010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shanbhogue AK, Karnad AB and Prasad SR:
Tumor response evaluation in oncology: Current update. J Comput
Assist Tomogr. 34:479–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thalgott M, Rack B, Eiber M, Souvatzoglou
M, Heck MM, Kronester C, Andergassen U, Kehl V, Krause BJ, Gschwend
JE, et al: Categorical versus continuous circulating tumor cell
enumeration as early surrogate marker for therapy response and
prognosis during docetaxel therapy in metastatic prostate cancer
patients. BMC cancer. 15:4582015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okabe H, Tsunoda S, Hosogi H, Hisamori S,
Tanaka E, Tanaka S and Sakai Y: Circulating tumor cells as an
independent predictor of survival in advanced gastric cancer. Ann
Surg Oncol. 22:3954–3961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Liang H, Yu T, Xie J, Chen S, Dong
H, Sinko PJ, Lian S, Xu J, Wang J, et al: Isolation and
characterization of living circulating tumor cells in patients by
immunomagnetic negative enrichment coupled with flow cytometry.
Cancer. 121:3036–3045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams DL, Stefansson S, Haudenschild C,
Martin SS, Charpentier M, Chumsri S, Cristofanilli M, Tang CM and
Alpaugh RK: Cytometric characterization of circulating tumor cells
captured by microfiltration and their correlation to the
cellsearch® CTC test. Cytometry A. 87:137–144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu M, Stott S, Toner M, Maheswaran S and
Haber DA: Circulating tumor cells: Approaches to isolation and
characterization. J Cell Biol. 192:373–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gazzaniga P, Raimondi C, Nicolazzo C,
Carletti R, di Gioia C, Gradilone A and Cortesi E: The rationale
for liquid biopsy in colorectal cancer: A focus on circulating
tumor cells. Expert Rev Mol Diagn. 15:925–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antolovic D, Galindo L, Carstens A,
Rahbari N, Büchler MW, Weitz J and Koch M: Heterogeneous detection
of circulating tumor cells in patients with colorectal cancer by
immunomagnetic enrichment using different EpCAM-specific
antibodies. BMC Biotechnol. 10:352010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heo CK, Hwang HM, Ruem A, Yu DY, Lee JY,
Yoo JS, Kim IG, Yoo HS, Oh S, Ko JH and Cho EW: Identification of a
mimotope for circulating anti-cytokeratin 8/18 antibody and its
usage for the diagnosis of breast cancer. Int J Oncol. 42:65–74.
2013.PubMed/NCBI
|
|
14
|
Wang Y, Zhu JF, Liu YY and Han GP: An
analysis of cyclin D1, cytokeratin 5/6 and cytokeratin 8/18
expression in breast papillomas and papillary carcinomas. Diagn
Pathol. 8:82013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strassen U, Hofauer B, Matsuba Y, Becker
K, Mansour N and Knopf A: Bronchogenic cancer: It still exists.
Laryngoscope. 2015.
|
|
16
|
Yin J, Wang Y, Yin H, Chen W, Jin G, Ma H,
Dai J, Chen J, Jiang Y, Wang H, et al: Circulating tumor cells
enriched by the depletion of leukocytes with bi-antibodies in
non-small cell lung cancer: Potential clinical application. PLoS
One. 10:e01370762015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaiopoulos AG, Kostakis ID, Gkioka E,
Athanasoula KCh, Pikoulis E, Papalambros A, Christopoulos P, Gogas
H, Kouraklis G and Koutsilieris M: Detection of circulating tumor
cells in colorectal and gastric cancer using a multiplex PCR assay.
Anticancer Res. 34:3083–3092. 2014.PubMed/NCBI
|
|
18
|
Meng S, Tripathy D, Frenkel EP, Shete S,
Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et
al: Circulating tumor cells in patients with breast cancer
dormancy. Clin Cancer Res. 10:8152–8162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Najjar F, Alammar M, Bachour M and
Al-Massarani G: Circulating endothelial cells as a biomarker in
non-small cell lung cancer patients: Correlation with clinical
outcome. Int J Biol Markers. 29:e337–e344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang X, Gao P, Sun J, Chen X, Song Y,
Zhao J, Xu H and Wang Z: Clinicopathological and prognostic
significance of circulating tumor cells in patients with gastric
cancer: A meta-analysis. Int J Cancer. 136:21–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Botteri E, Sandri MT, Bagnardi V, Munzone
E, Zorzino L, Rotmensz N, Casadio C, Cassatella MC, Esposito A,
Curigliano G, et al: Modeling the relationship between circulating
tumour cells number and prognosis of metastatic breast cancer.
Breast Cancer Res Treat. 122:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Court CM, Ankeny JS, Hou S, Tseng HR and
Tomlinson JS: Improving pancreatic cancer diagnosis using
circulating tumor cells: Prospects for staging and single-cell
analysis. Expert Rev Mol Diagn. 15:1491–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang L, Zhao S, Liu W, Parchim NF, Huang
J, Tang Y, Gan P and Zhong M: Diagnostic accuracy of circulating
tumor cells detection in gastric cancer: Systematic review and
meta-analysis. BMC Cancer. 13:3142013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cristofanilli M: Circulating tumor cells,
disease progression and survival in metastatic breast cancer. Semin
Oncol. 33:(Suppl 9). S9–S14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camara O, Rengsberger M, Egbe A, Koch A,
Gajda M, Hammer U, Jörke C, Rabenstein C, Untch M and Pachmann K:
The relevance of circulating epithelial tumor cells (CETC) for
therapy monitoring during neoadjuvant (primary systemic)
chemotherapy in breast cancer. Ann Oncol. 18:1484–1492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Das A, Kunkel M, Joudeh J, Dicker DT,
Scicchitano A, Allen JE, Sarwani N, Yang Z, Kaifi J, Zhu J, et al:
Clinico-pathological correlation of serial measurement of
circulating tumor cells in 24 metastatic colorectal cancer patients
receiving chemotherapy reveals interpatient heterogeneity
correlated with CEA levels but independent of KRAS and BRAF
mutation. Cancer Biol Ther. 16:709–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsushita D, Uenosono Y, Arigami T,
Yanagita S, Nishizono Y, Hagihara T, Hirata M, Haraguchi N, Arima
H, Kijima Y, et al: Clinical significance of circulating tumor
cells in peripheral blood of patients with esophageal squamous cell
carcinoma. Ann Surg Oncol. 22:3674–3680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang X, Gao P, Song Y, Sun J, Chen X,
Zhao J, Liu J, Xu H and Wang Z: Relationship between circulating
tumor cells and tumor response in colorectal cancer patients
treated with chemotherapy: A meta-analysis. BMC Cancer. 14:9762014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubisch I, de Albuquerque A, Schuppan D,
Kaul S, Schaich M and Stölzel U: Prognostic role of a multimarker
analysis of circulating tumor cells in advanced gastric and
gastroesophageal adenocarcinomas. Oncology. 89:294–303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fei F, Du Y, Di G, Wu J and Shao Z: Are
changes in circulating tumor cell (CTC) count associated with the
response to neoadjuvant chemotherapy in local advanced breast
cancer? A meta-analysis. Oncol Res Treat. 37:250–254. 2014.
View Article : Google Scholar : PubMed/NCBI
|