Introduction
Acute leukemias are clonal malignant disorders
arising from the primitive pluripotent hematopoietic cell,
characterized by impaired proliferation of leukemic progenitors
(1,2). They are characterized by recurring
chromosomal aberrations and gene mutations, with contribution of
epigenetic modifications (3), which
are crucial in differentiation, proliferation and survival
pathways.
According to the latest Globocan data published in
2012, 351,965 individuals worldwide were diagnosed with leukemia
(chronic and acute, as well as lymphoid and myeloid) and 265,491
succumbed to this disease. A marginal male predominance was also
reported by the World Health Organization (WHO), with a male:female
ratio of ~1.4 (http://globocan.iarc.fr/Default.aspx).
The diagnosis is based on bone marrow analysis: a
smear morphology with a blast count >20% or the presence of
recurring cytogenetic abnormalities [t(8,21),
inv16, t(16,16), or t(15,17)]
confirms the diagnosis (4).
The classification of acute leukemia, previously
based only on morphological and cytochemistry findings, from 2002
onwards it includes phenotypic aspects, as well as cytogenetic and
molecular characteristics, which are of well-known prognostic value
(4).
over the last 30 years, conventional treatment has
been based on the combination of anthracyclines with cytarabine
(5). The outcome depends on multiple
characteristics, including karyotype (6–9),
response to the induction regimen (10), age and comorbid conditions (11). AML originating de novo vs.
therapy-related, was also associated with outcome. As cytogenetics
is considered the single most important prognostic marker,
predicting remission, relapse and overall survival (OS), several
cooperative group trials, including the United Kingdom Medical
Research Council, Southwest Oncology Group (SWOG), Eastern
Cooperative Oncology Group and Cancer and Leukemia Group B,
designed risk scores based on large cohorts of patients (6,8,9,12),
dividing patients in four groups, namely favorable, intermediate,
unfavorable and unknown, based on the cytogenetic findings.
However, despite these efforts, the cure rates
remain disappointing, with complete response rates at first
induction of 60–80% (age <60 years), but with cure rates of
30–40% (13).
The aim of the present study was to investigate the
Portuguese AML population and assess the effect of
cytogenetics.
Patients and methods
Study design
A retrospective observational study was conducted,
including AML patients aged <65 years, diagnosed and treated at
the Portuguese Institute of Oncology (Porto, Portugal), between
January, 2002 and December, 2010.
Patients
Between 2002 and 2010, a total of 225 patients were
diagnosed with AML in our department. Following exclusion of
patients aged >65 years and cases with acute promyelocytic
leukemia, 128 patients were selected for inclusion in this
cohort.
Data regarding gender, age, date of diagnosis, WHO
classification, cytogenetic abnormalities, SWOG pretreatment risk
score (6), first- and further-line
treatment, response, consolidation, relapse, date of relapse, bone
marrow transplantation (allogeneic or autologous), date of last
hospital visit or date and cause of death, were obtained from the
medical records of previously selected patients and retrospectively
reviewed.
Diagnosis was based on standard criteria (4,14,15). All
cases initially classified according to the French American British
classification (14) were reviewed
and reclassified according to WHO (4). A blast count >20% confirmed the
diagnosis of AML.
Specimens were collected for morphological,
immunophenotypic and genetic analyses (cytogenetic and molecular
biology).
The patients were treated with the classic
idarubicin and cytarabine combination (16,17) or
with daunorubicin, cytarabine and cyclosporine (18,19) if
bone marrow dysplasia was present.
The response criteria used (complete remission,
failure or relapse) were defined by international working groups
(4,15,20,21).
This investigation was performed in accordance with
the principles of the Declaration of Helsinki and was approved by
the local Ethics Committee.
Cytogenetics
Standard G-banding was performed on patient bone
marrow samples using standard techniques; the cytogenetic results
were presented according to the International System for Human
Cytogenetic Nomenclature (22). The
patients were then analysed according to the cytogenetic risk
subgroups defined by the SWOG (6).
Statistical analysis
Analysis of data was performed using the SPSS
statistical software for Windows, version 17.0 (SPSS Inc., Chicago,
IL, USA). Differences in proportions were evaluated by the
Chi-square test. The probabilities of survival were calculated, and
the means and life tables were computed using the product limit
estimate of the Kaplan-Meier method and analysed using the Breslow
(generalized Wilcoxon) test, a statistical test for equality of
survival distributions. P<0.05 was considered to indicate
statistically significant differences. Hazard ratio was also
accessed using a multivariate Cox regression analysis for 3- and
5-year OS.
Survival duration was defined as the time between
diagnosis and death, or the time of the last clinical
evaluation.
As defined, prognostic factor is a measurement that
is associated with clinical outcome in the absence of therapy or
with the application of a standard therapy; predictive factor is a
measurement that is associated with response or lack of response to
a particular therapy (23,24).
Results
Patient cohort
Between 2002 and 2010, a total of 225 patients were
diagnosed with aml at the Portuguese Institute of Oncology (Porto,
Portugal); of those, 128 were aged <65 years. Both genders were
equally represented with 64 patients. The median age at diagnosis
was 54 years (range, 17–65 years). Therapy-related AML constituted
23.1% of all cases.
Karyotype analysis was performed in all patients; in
2.3% of the cases (n=3), there were insufficient metaphases for
karyotype analysis. A normal karyotype was the most frequent result
(n=54; 43.2%). Core-binding factor anomalies were detected in 17
karyotypes (13.6%). Anomalies involving chromosomes 5, 7 and 8 were
detected in 18 cases (14.4%); a complex karyotype was observed in
9.6% (n=12) of the studied cases. Other less frequent alterations
were detected in the remaining 24 cases (19.2%).
Using the SWOG pretreatment risk criteria system,
the intermediate group included the majority of the patients
(55.5%; n=71), whereas 37 patients (28.9%) had an unfavorable risk
and 17 (13,6%) were included in the favorable group.
There was a statistically significant association
between cytogenetic groups and age (P=0.031), gender [P=0.025; risk
ratio (RR)=3.632; 95% confidence interval (CI): 1.113–11.852],
indication for allogeneic stem cell transplantation (P=0.023;
RR=1.317; 95% CI: 1.184–1.465), complete response achievement
(P=0.013; RR=1.385; 95% CI: 1.232–1.556) and relapse (P=0.048;
RR=3.181; 95% CI: 0.966–10.478) (Table
I). A significant association was also observed with global OS
(P=0.003) and 5-year OS (P=0.001).
 | Table I.Characterization of the complete
cohort and of the 3 different SWOG pretreatment risk criteria
groups. |
Table I.
Characterization of the complete
cohort and of the 3 different SWOG pretreatment risk criteria
groups.
|
|
| SWOG pretreatment
risk criteria, n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Factors | Total, 128
(100.0) | Favorable, 17
(13.3) | Intermediate, 71
(55.5) | Unfavorable, 37
(28.9) | Unknown, 3 (2.3) | P-valuea | RRa (95% CI) |
|---|
| Median age
(years) | 54 | 44 | 52 | 53 | 54 | 0.043b | – |
| Female gender | 64 (50.0) | 13 (76.5) | 34 (47.9) | 17 (45.9) | 0 (0.0) | 0.019b | 3.82
(1.07–14.92) |
| t-AML | 24 (23.1) | 2 (11.8) | 11 (15.5) | 11 (29.7) | 0 (0.0) | 0.428b | 1.85
(0.36–12.69) |
| Treatment
(‘7+3’). | 87 (68.0) | 15 (88.2) | 49 (69.0) | 22 (59.5) | 1 (33.3) | 0.054b | 4.06
(0.82–27.17) |
| Consolidation | 96 (86.5) | 17 (100.0) | 57 (86.4) | 20 (54.1) | 2 (66.6) | 0.248b | 3.23
(0.40–69.84) |
| Allogeneic stem
cell | 26 (20.3) | 0 (0.0) | 16 (22.5) | 10 (27.0) | 0 (0.0) | 0.084b | 0.10
(0.01–1.49) |
| Complete
response | 111 (86.7) | 17 (100.0) | 66 (93.0) | 26 (70.3) | 2 (66.6) | 0.041b | 6.59
(0.86–138.32) |
| Relapse | 52 (46.5) | 4 (23.5) | 33 (50.0) | 13 (48.1) | 2 (100.0) | 0.040b | 0.30
(0.08–1.09) |
| OS (months) | 24 | NR | 20 | 14 | 2.4 | 0.006c | – |
| 5-year OS (%) | 44.5 | 80.9 | 31.3 | 29.4 | 0.0 | 0.003c | – |
Treatment
Complete remission was achieved in 86.7% (n=111) of
the cases. Relapse occurred in 52 patients (46.8% of the
responders; 46.5% of the total population).
Consolidation followed induction with at least one
chemotherapy course (median of 4 courses; range, 1–5) in 96
patients (86.5%). All patients from the favorable group followed
treatment after induction; 86.4% (n=57) and 54.1% (n=20) of the
patients from the intermediate and unfavorable groups,
respectively, received consolidation therapy with hight dose
cytarabine.
Allogeneic stem cell bone marrow transplantation was
performed in 26 patients. The majority of the transplants (61.5%)
were performed in the intermediate group (n=16).
Outcome
The median survival including all patients was 24
months. The OS at 3 and 5 years was 48.4 and 44.5%, respectively.
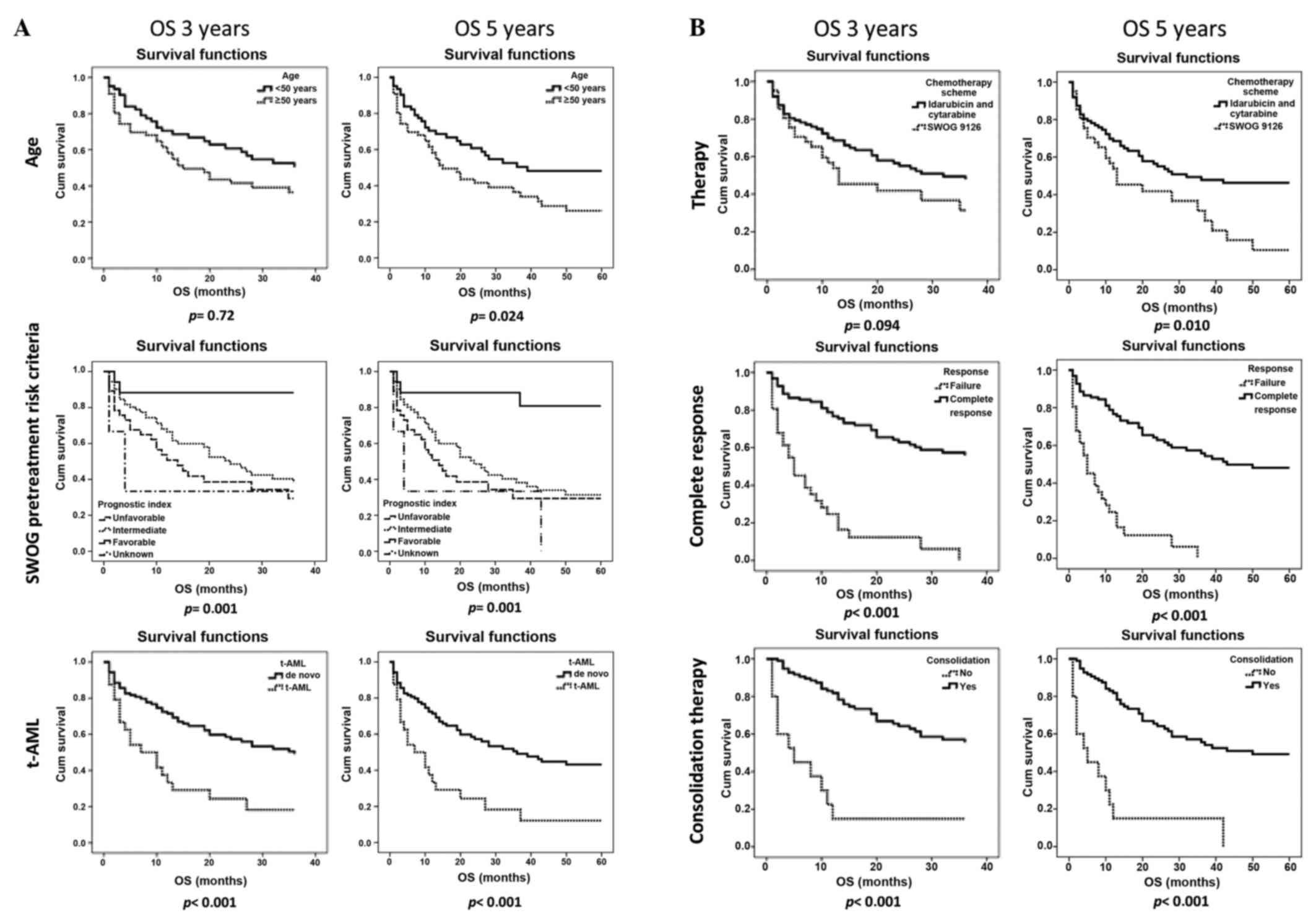
Different factors were found to statistically significantly affect
OS at 3 and 5 years, such as complete response achievement
(P<0.001 for both), SWOG pretreatment risk criteria (P=0.001 for
both), presence of therapy-related AML (P<0.001 for both) and
consolidation therapy (P<0.001 for both). Age (P=0.024) and
therapy (P=0.01) only affected 5-year OS (Fig. 1).
A multivariate Cox regression analysis (Table II) demonstrated that OS at 3 and 5
years was affected by the same factors: Achievement of complete
response (P=0.011; RR=0.385; 95% CI: 0.184–0.806; and P=0.012;
RR=0.388; 95% CI: 0.597–1.994, respectively), therapy-related AML
(P=0.016; RR=2.756; 95% CI: 0.486–1.281; and P=0.031; RR=2.369; 95%
CI: 1.081–5.189, respectively) and consolidation therapy (P=0.005;
RR=0.328; 95% CI: 0.150–0.720; and P=0.002; RR=0.308; 95% CI:
0.144–0.657, respectively). Other studied variables, such as
gender, age (<50 or >50 years), therapy and SWOG pretreatment
risk criteria did not significantly affect outcome.
 | Table II.Multivariable analysis for 5-year
overall survival. |
Table II.
Multivariable analysis for 5-year
overall survival.
|
| 5-year overall
survival |
|---|
|
|
|
|---|
| Factors |
P-valuea | HRb (95% CI) |
|---|
| Female gender | 0.293 | 1.376
(0.759–2.494) |
| Age | 0.627 | 1.006
(0.981–1.031) |
| Complete
response | 0.019 | 0.421
(0.204–0.866) |
| Treatment | 0.540 | 1.053
(0.893–1.242) |
| SWOG pretreatment
risk criteria | 0.074 | 1.724
(0.949–3.134) |
| t-AML | 0.019 | 2.404
(1.152–5.017) |
| Consolidation
therapy | 0.005 | 0.342
(0.161–0.727) |
Discussion
To the best of our knowledge, this study is the
first to investigate and characterize Portuguese patients diagnosed
with AML and their outcome.
In our study, there were more therapy-related AML
cases compared with other published series (25). As our hospital is an oncology centre,
it admits a high number of cancer patients. when patients develop
AML as a secondary malignancy, they are referred to our
department.
The most frequent cytogenetic finding was a normal
karyotype (26,27). Data on molecular findings were not
available for this study. NPM1 and FLT3 gene mutations are now
relevant for prognosis assessment (28–32).
However, when the patients of this cohort were diagnosed, this
molecular analysis was not widely available.
Using the SWOG pretreatment risk criteria, three
different groups may be defined, with different survivals. Male
gender, failure of complete response achievement, risk of relapse
and indication for allogeneic bone marrow transplantation are
significantly associated with an unfavorable cytogenetic group.
Analysing the effect of age, response to induction, treatment
administered, consolidation therapy and t-AML on OS at 3 and 5
years, we observed that all these factors affect outcome.
Cytogenetic risk, defined by the SWOG pretreatment risk criteria,
also affects OS. However, when analysing this score in association
with other factors also related to outcome, such as complete
response achievement, cytogenetics does not statistically
significantly affect OS. This suggests that cytogenetic groups
previously defined and related to disease prognosis (7,12,33,34)
may be factors predictive of response, being statistically relevant
for complete response to induction therapy, but not prognostic
factors, having no statistical significance in OS when associated
with other entities.
Predictive and prognostic factor are definitions
frequently confused and overlapping. As defined in the literature,
a prognostic factor is a measurement that is associated with
clinical outcome in the absence of therapy or with the application
of a standard therapy that patients are likely to receive; a
predictive factor is a measurement that is associated with response
or lack of response to a particular therapy (23,24). In
conclusion, SWOG pretreatment risk groups may not be consistent as
prognostic markers, but may be more reliable as predictive markers
for response to standard therapy, while complete response
achievement is a prognostic markers as it affects outcome.
References
|
1
|
Becker MW and Jordan CT: Leukemia stem
cells in 2010: Current understanding and future directions. Blood
Rev. 25:75–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giles FJ, Keating A, Goldstone AH, Avivi
I, Willman CL and Kantarjian HM: Acute myeloid leukemia. Hematology
Am Soc Hematol Educ Program. 73–110. 2002.PubMed/NCBI
|
|
3
|
Chen J, Odenike O and Rowley JD:
Leukemogenesis: More than mutant genes. Nat Rev Cancer. 10:23–36.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yates JW, Wallace HJ Jr, Ellison RR and
Holland JF: Cytosine arabinoside (NSC-63878) and daunorubicin
(NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer
Chemother Rep. 57:485–488. 1973.PubMed/NCBI
|
|
6
|
Slovak ML, Kopecky KJ, Cassileth PA,
Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR,
Rowe JM, et al: Karyotypic analysis predicts outcome of
preremission and postremission therapy in adult acute myeloid
leukemia: A southwest oncology group/eastern cooperative oncology
group study. Blood. 96:4075–4083. 2000.PubMed/NCBI
|
|
7
|
Grimwade D, Hills RK, Moorman AV, Walker
H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ and Burnett
AK: National Cancer Research Institute Adult Leukemia Working
Group: Refinement of cytogenetic classification in acute myeloid
leukemia: Determination of prognostic significance of rare
recurring chromosomal abnormalities among 5876 younger adult
patients treated in the United Kingdom medical research council
trials. Blood. 116:354–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grimwade D, Walker H, Harrison G, Oliver
F, Chatters S, Harrison CJ, Wheatley K, Burnett AK and Goldstone
AH: Medical Research Council Adult Leukemia Working Party: The
predictive value of hierarchical cytogenetic classification in
older adults with acute myeloid leukemia (AML): Analysis of 1065
patients entered into the United Kingdom medical research council
AML11 trial. Blood. 98:1312–1320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byrd JC, Mrózek K, Dodge RK, Carroll AJ,
Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS,
et al: Pretreatment cytogenetic abnormalities are predictive of
induction success, cumulative incidence of relapse and overall
survival in adult patients with de novo acute myeloid leukemia:
Results from cancer and leukemia group B (CALGB 8461). Blood.
100:4325–4336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walter RB, Kantarjian HM, Huang X, Pierce
SA, Sun Z, Gundacker HM, Ravandi F, Faderl SH, Tallman MS,
Appelbaum FR and Estey EH: Effect of complete remission and
responses less than complete remission on survival in acute myeloid
leukemia: A combined Eastern cooperative oncology group, southwest
oncology group and M. D. Anderson cancer center study. J Clin
Oncol. 28:1766–1771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jabbour EJ, Estey E and Kantarjian HM:
Adult acute myeloid leukemia. Mayo Clin Proc. 81:247–260. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grimwade D, Walker H, Oliver F, Wheatley
K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A and
Goldstone A: The importance of diagnostic cytogenetics on outcome
in AML: Analysis of 1,612 patients entered into the MRC AML 10
trial. The medical research council adult and children's leukemia
working parties. Blood. 92:2322–2333. 1998.PubMed/NCBI
|
|
13
|
Odenike O, Thirman MJ, Artz AS, Godley LA,
Larson RA and Stock W: Gene mutations, epigenetic dysregulation and
personalized therapy in myeloid neoplasia: Are we there yet? Semin
Oncol. 38:196–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bennet JM, Catovsky D, Daniel MT, Flandrin
G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute myeloid leukemias:
French-American-British cooperative group. Br J Hematol.
33:451–458. 1976. View Article : Google Scholar
|
|
15
|
British Committee for Standards in
Hematology; Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D,
Craddock C, Kell J, Homewood J, Campbell K, et al: Guidelines on
the management of acute myeloid leukemia in adults. Br J Hematol.
135:450–474. 2006. View Article : Google Scholar
|
|
16
|
Wiernik PH, Banks PL, Case DC Jr, Arlin
ZA, Periman PO, Todd MB, Ritch PS, Enck RE and Weitberg AB:
Cytarabine plus idarubicin or daunorubicin as induction and
consolidation therapy for previously untreated adult patients with
acute myeloid leukemia. Blood. 79:313–319. 1992.PubMed/NCBI
|
|
17
|
Wiernik PH, Case DC Jr, Periman PO, Arlin
ZA, Weitberg AB, Ritch PS and Todd MB: A multicenter trial of
cytarabine plus idarubicin or daunorubicin as induction therapy for
adult nonlymphocytic leukemia. Semin Oncol. 16(1): Suppl 2.
S25–S29. 1989.
|
|
18
|
List AF, Spier C, Greer J, Wolff S, Hutter
J, Dorr R, Salmon S, Futscher B, Baier M and Dalton W: Phase I/II
trial of cyclosporine as a chemotherapy-resistance modifier in
acute leukemia. J Clin Oncol. 11:1652–1660. 1993.PubMed/NCBI
|
|
19
|
List AF, Kopecky KJ, Willman CL, Head DR,
Persons DL, Slovak ML, Dorr R, Karanes C, Hynes HE, Doroshow JH, et
al: Benefit of cyclosporine modulation of drug resistance in
patients with poor-risk acute myeloid leukemia: A southwest
oncology group study. Blood. 98:3212–3220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the international working
group for diagnosis, standardization of response criteria,
treatment outcomes and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonzalez Garcia JR and Meza-Espinoza JP:
Use of the international system for human cytogenetic nomenclature
(ISCN). Blood. 108:3952–3953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clark GM: Prognostic factors versus
predictive factors: Examples from a clinical trial of erlotinib.
Mol Oncol. 1:406–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clark GM, Zborowski DM, Culbertson JL,
Whitehead M, Savoie M, Seymour L and Shepherd FA: Clinical utility
of epidermal growth factor receptor expression for selecting
patients with advanced non-small cell lung cancer for treatment
with erlotinib. J Thorac Oncol. 1:837–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suvajdžić N, Cvetković Z, Dorđević V,
Kraguljac-Kurtović N, Stanisavljević D, Bogdanović A, Djunić I,
Colović N, Vidović A, Elezović I and Tomin D: Prognostic factors
for therapy-related acute myeloid leukemia (t-AML) - a single
centre experience. Biomed Pharmacother. 66:285–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Farag SS, Ruppert AS, Mrózek K, Mayer RJ,
Stone RM, Carroll AJ, Powell BL, Moore JO, Pettenati MJ, Koduru PR,
et al: Outcome of induction and postremission therapy in younger
adults with acute myeloid leukemia with normal karyotype: A cancer
and leukemia group B study. J Clin Oncol. 23:482–493. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Medeiros BC: Comparing apples and oranges
in normal karyotype acute myeloid leukemia. J Clin Oncol.
27:4742009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christiansen DH and Pedersen-Bjergaard J:
Internal tandem duplications of the FLT3 and MLL genes are mainly
observed in atypical cases of therapy-related acute myeloid
leukemia with a normal karyotype and are unrelated to type of
previous therapy. Leukemia. 15:1848–1851. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bienz M, Ludwig M, Leibundgut EO, Mueller
BU, Ratschiller D, Solenthaler M, Fey MF and Pabst T: Risk
assessment in patients with acute myeloid leukemia and a normal
karyotype. Clin Cancer Res. 11:1416–1424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Medeiros BC, Gotlib J and Zehnder J:
Molecular stratification of patients with normal karyotype acute
myeloid leukemia based on initial assessment of FLT3-internal
tandem duplication status at first complete remission. Leuk
Lymphoma. 50:851–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medeiros BC: Role of CEBPA in normal
karyotype acute myeloid leukemia. J Clin Oncol. 27:21052009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshimoto G, Nagafuji K, Miyamoto T,
Kinukawa N, Takase K, Eto T, Kato K, Hayashi S, Kamimura T, Ohno Y,
et al: FLT3 mutations in normal karyotype acute myeloid leukemia in
first complete remission treated with autologous peripheral blood
stem cell transplantation. Bone Marrow Transplant. 36:977–983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Foran JM: New prognostic markers in acute
myeloid leukemia: Perspective from the clinic. Hematology Am Soc
Hematol Educ Program. 2010:47–55. 2010.PubMed/NCBI
|
|
34
|
Kadia T, Kantarjian H, Garcia-Manero G,
Borthakur G, Wang X, Patel K, Jabbour E, Brandt M, Daver N,
Pemmaraju N, et al: Prognostic significance of the medical research
council cytogenetic classification compared with the European
LeukemiaNet risk classification system in acute myeloid leukemia.
Br J Hematol. 170:590–593. 2015. View Article : Google Scholar
|