Introduction
Giant cell tumor of bone (GCTB) constitutes ~5% of
all bone tumors (1). Aggressive GCTB
presents as a bulging bone mass. Although treatable with surgical
curettage followed by adjuvant therapy, such as liquid nitrogen and
phenol, the recurrence rate of GCTB is high (2–4). Since
curettage of GCTB in the sacral vertebrae is difficult due to its
location, en bloc resection is preferred to prevent recurrence.
However, surgery in that site is difficult, and there are issues
such as intraoperative bleeding and postoperative dysfunction.
Furthermore, the surgical results are not always satisfactory and
the recurrence rates for sacral GCTB cannot be reduced through
adjuvant therapy. Therefore, conservative treatment must be
considered as an option (5).
Although radiotherapy is effective for sacral GCTB,
there is a risk of inducing a secondary malignant bone tumor
(6). Alternatively, several studies
have demonstrated that selective arterial embolization may reduce
and control tumors, and is an effective treatment for preserving
function (7–9). In addition, infusion of the
aminobisphosphonate zoledronic acid (ZOL) reportedly inhibits bone
destruction at inoperable sites and prevents recurrence (10–12).
While treatments with selective arterial embolism and ZOL are
normally performed independently, patients who do not respond to
these treatments require other measures.
The anti-receptor activator of nuclear factor κ-B
ligand (anti-RANKL) monoclonal antibody, denosumab, was recently
released. Denosumab characteristically plays a role in preventing
osteolysis by inhibiting the function of osteoclast-like giant
cells that are present in GCTB (13–15). In
fact, the efficacy of denosumab against GCTB has been evaluated in
a number of studies (13,16,17);
however, there has been no report of a patient receiving all three
treatments (selective arterial embolization, ZOL and denosumab) to
date.
We herein report a case of a patient with sacral
GCTB showing a poor response to combination therapy with arterial
embolization and ZOL, who was subsequently treated with
denosumab.
Case report
A 32-year-old woman visited a local clinic with a
5-month history of progressive buttock pain and numbness in the
bilateral posterior thighs. The patient had a history of hepatitis
B. The physical findings included tenderness in the buttocks and
difficulty walking due to persistent buttock pain in the sitting as
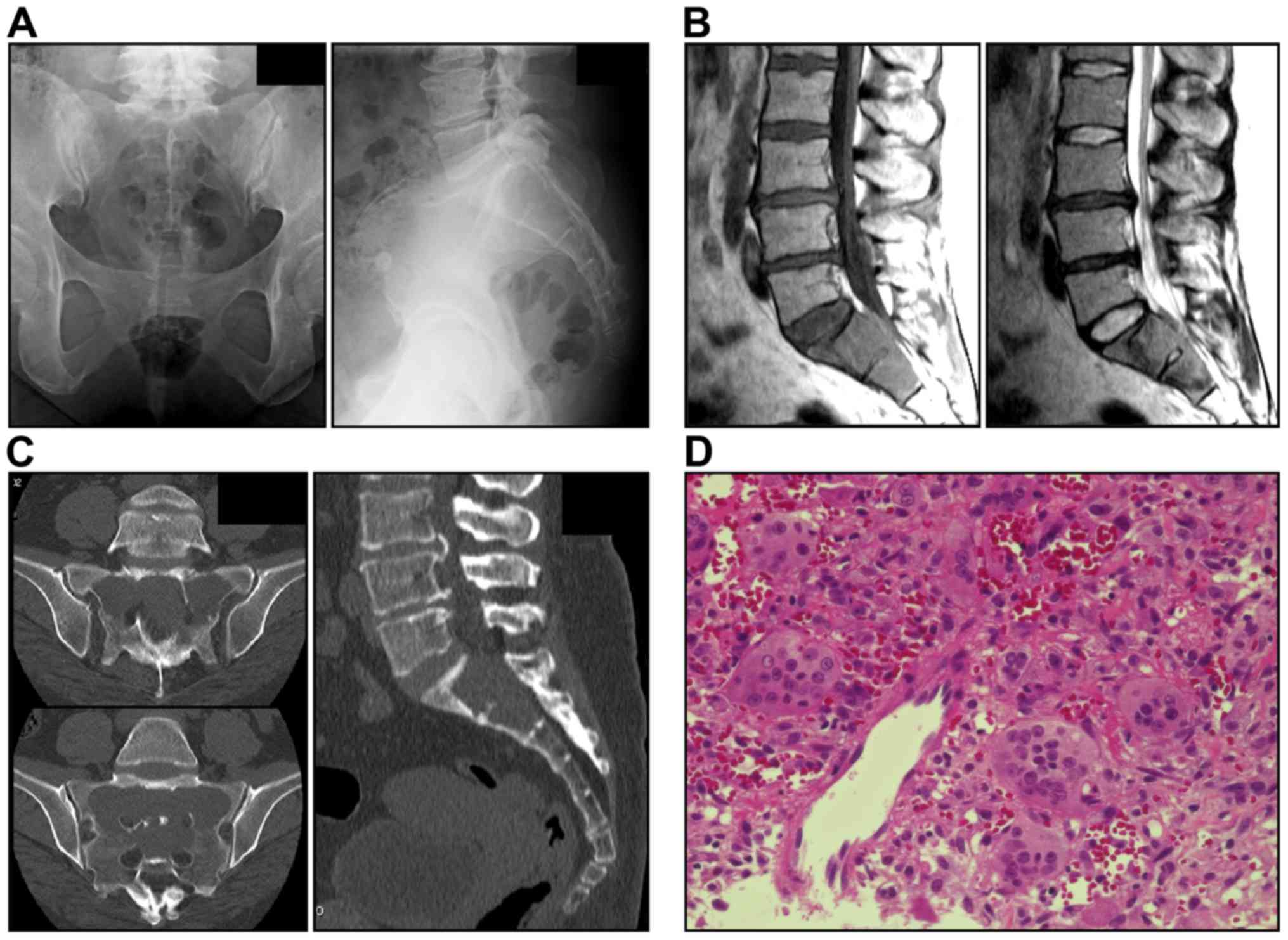
well as standing positions. Radiography revealed osteolysis in the
entire S1-S2 region (Fig. 1A).
Magnetic resonance imaging revealed a bulging heterogeneous solid
lesion (Fig. 1B). Computed
tomography (CT) scans revealed an inner structure without a septal
wall and thinning of the surrounding cortical bone (Fig. 1C). The imaging findings were
suggestive of an aggressive bone tumor and GCTB was diagnosed by
CT-guided bone biopsy (Fig. 1D).
The patient was informed on the risks of surgery and
recurrence rates, provided informed consent and opted for
conservative therapy. First, selective arterial embolization was
performed by a radiologist. The embolization was performed using
Gelfoam® (Pfizer Co. New York, NY, USA) once a month for
3 months, but there was no improvement of the clinical symptoms
after three procedures, and a hypervascular tumor stain was
observed. The response was considered to be poor and intravenous
infusion of 4 mg ZOL was initiated concurrently. ZOL was
administered once a month, 2 weeks after each arterial
embolization. This combination was repeated for 10 months, but the
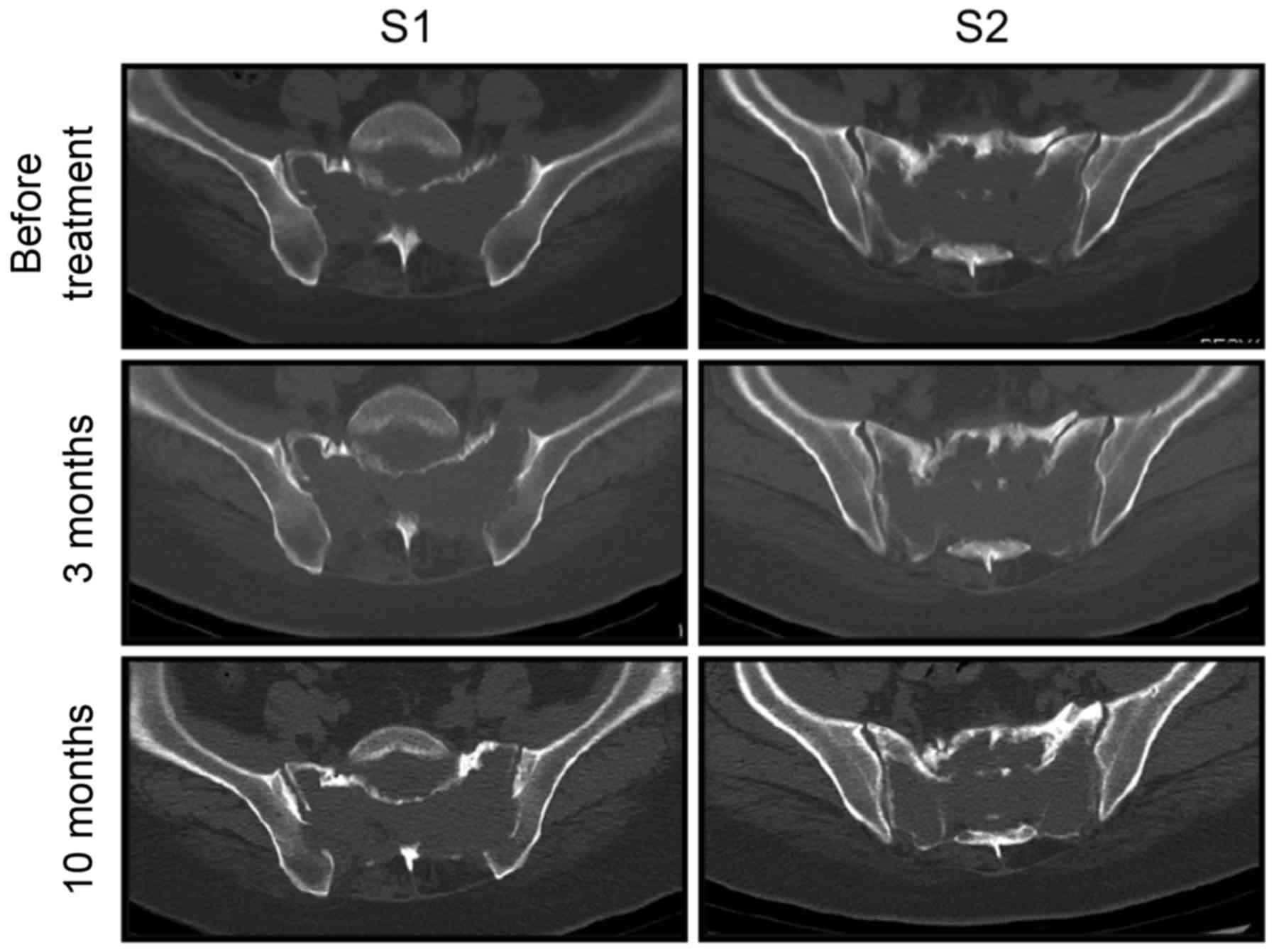
pain did not subside and daily activities were impaired. In
addition, CT scans did not reveal distinct bone sclerosis of the
sacral cortex (Fig. 2). Therefore,
these treatments were discontinued and denosumab was initiated.
The patient received monthly injections of denosumab
120 mg subcutaneously, in addition to daily doses of calcium 610
mg, vitamin D 400 IU and magnesium 30 mg orally. The pain had
drastically decreased by the third dose of denosumab, and pain on
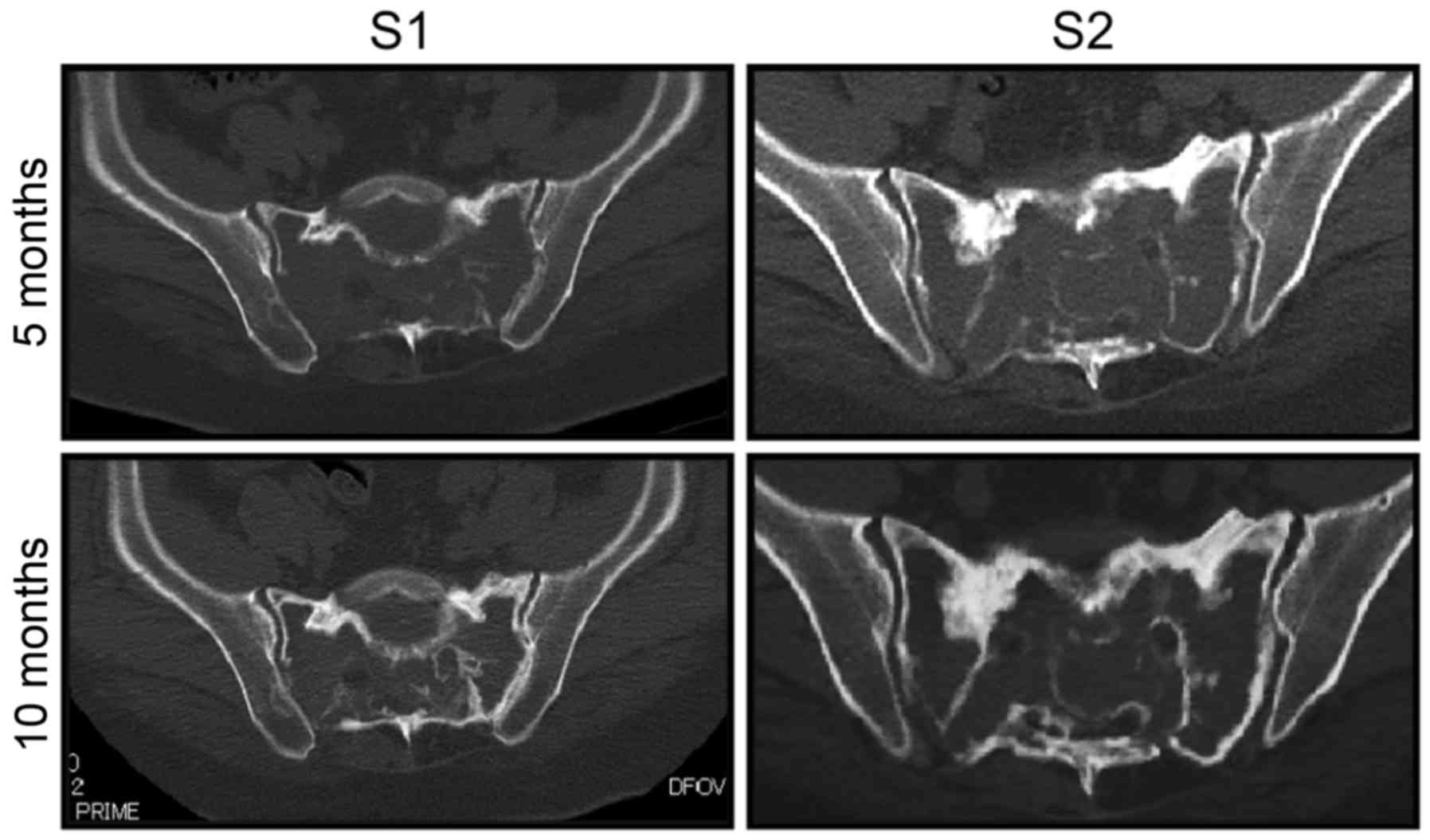
sitting had completely resolved. CT scans taken 5 and 10 months
after the initiation of denosumab revealed gradual appearance of
bone sclerosis around the sacrum in the transverse and sagittal
planes (Fig. 3). Unfortunately, the
patient had a history of hepatitis B and developed hepatic
impairment. Hence, denosumab was discontinued after 12 months and
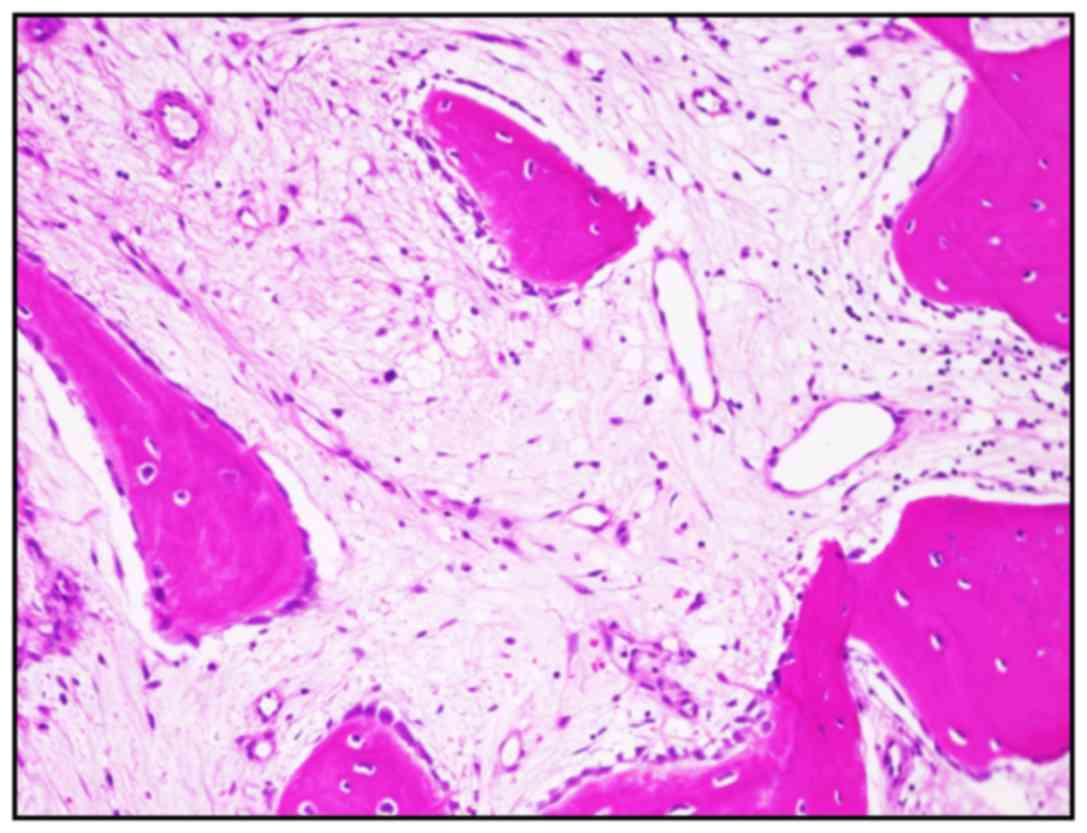
treatment for hepatitis was initiated. At that time, a CT-guided
needle biopsy was performed to determine the efficacy of our
treatment strategy. The tissues displayed remodeled bone formation
and a partly fibrous stroma, but no functional stromal cell or
osteoclast-like giant component was identified (Fig. 4). The laboratory findings were also
noteworthy. The blood levels of tartrate-resistant acid phosphatase
5b (TRACP-5b) were monitored throughout the course of treatment.
During combination therapy with arterial embolization and ZOL, the
TRACP-5b level was 1,423 mU/dl (normal range, 120–420 mU/dl); by
the time denosumab was discontinued, the level had decreased to 233
mU/dl.
Two years after treatment discontinuation, the
patient remains symptom-free, shows no radiographic progression of
the disease and has not required surgery.
Written informed consent for the publication of this
case report and related images was obtained from the patient.
Discussion
The standard treatment for GCTB is surgery,
including adjuvant treatment with liquid nitrogen and ethanol at
the time of curettage. However, the recurrence rate with or without
adjuvant therapy, particularly for GCTB in the sacral vertebrae, is
high. Therefore, practitioners must be cautious when deciding on
surgical intervention (15). In the
present case, conservative therapy was selected to prevent
functional impairment.
Arterial embolization and treatment with
bisphosphonates are reportedly effective for patients with sacral
GCTB. Lin et al (7) reported
that tumors were controlled and symptoms improved in 14 of 18
patients, although long-term follow-up to monitor for recurrence
was recommended. As in other cases, vascular embolization was
performed in our patient. However, the condition did not improve;
therefore, ZOL was added to improve efficacy. However, there was no
shell formation around the tumor, and angiography revealed a tumor
stain. Thus, therapy was switched to denosumab. The TRACP-5b
levels, which have recently been shown to be effective for
monitoring GCTB (18), began to
decrease after the initiation of denosumab and did not increase
after discontinuation of treatment. The tumor did not progress and
was deemed stable.
The reasons for our observations may be explained as
follows: GCTB consists of osteoclast-like giant cells and oval
mononuclear cells (stromal cells). Osteoclast-like giant cells and
their precursor cells express receptor activator of nuclear factor
κ-B (RANK), whereas oval mononuclear cells express RANK ligand
(RANKL) (19), which regulates
osteoclast function and plays a role in the formation, function and
survival of osteoclasts. Theoretically, bisphosphonates regulate
GCTB by reducing the number of osteoclasts and inhibiting
osteolysis, and they are reportedly effective for the treatment of
GCTB at sites that are difficult to operate (10). For example, a study by Lau et
al (20) comparing the antitumor
effect of ZOL (a bisphosphonate) with denosumab (another
bisphosphonate) on GCTB stromal cells in vitro demonstrated
that ZOL acts against tumors in a dose-dependent manner.
Furthermore, denosumab has little impact on cell viability, as it
does not directly block the RANKL signaling pathway in
osteoclast-like giant cells.
By contrast, denosumab was developed as a
RANKL-targeted antibody in the field of osteoporosis. Unlike
traditional bisphosphonates that induce apoptosis of osteoclasts by
adsorbing to the bone surface, denosumab inhibits the formation of
osteoclasts and impedes their function and survival (21). Since denosumab is present in body
fluids, it enters the haversian canal independent of body surface
area, and it may completely inhibit bone remodeling and improve the
porosity of cortical bone and the volume of cancellous bone. As
such, denosumab has been proven to act on both cortical and
cancellous bone (22).
Finally, bone destruction in GCTB is caused by
infiltration of osteoclast-like giant cells into the bone. While
ZOL exerts antitumor effects on osteoclast-like giant cells through
its accumulation in the bone, denosumab is effective in tumors
without bone infiltration, due to humoral immunity. Therefore,
denosumab may act more extensively on tumors.
To the best of our knowledge, no report has compared
the efficacy of ZOL with that of denosumab in a single patient to
date. The present case demonstrated the efficacy of denosumab.
However, as there have been no long-term studies of patients with
unresectable tumors controlled with denosumab, future researchers
may wish to investigate when surgery should be added.
In conclusion, we reported a case of a patient
responding poorly to combination therapy with arterial embolization
and ZOL, a treatment traditionally considered effective for sacral
GCTB. Our patient was instead successfully treated with denosumab.
To the best of our knowledge, this is the first report of a patient
who received arterial embolization, ZOL and denosumab in stages,
resulting in a positive clinical response to denosumab.
Acknowledgements
We would like to thank Honyaku Center Inc. for the
English editing of the manuscript.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
GCTB
|
giant cell tumor of bone
|
|
RANK
|
receptor activator of nuclear factor
κ-B
|
|
RANKL
|
receptor activator of nuclear factor
κ-B ligand
|
|
TRACP-5b
|
tartrate-resistant acid phosphatase
5b
|
|
ZOL
|
zoledronic acid
|
References
|
1
|
Fletcher CDM, Unni KK and Mertens F:
Pathology and genetics of tumours of soft tissue and bone. Lyon:
IARC. 2002.
|
|
2
|
Balke M, Ahrens H, Streitbuerger A,
Koehler G, Windelmann W, Gosheger G and Hardes J: Treatment options
for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol.
135:149–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durr HR, Maier M, Jansson V, Baur A and
Refior HJ: Phenol as an adjuvant for local control in the treatment
of giant cell tumor of the bone. Eur J Surg Oncol. 25:610–618.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malawer MM, Bickels J, Meller I, Buch RG,
Henshaw RM and Kollender Y: Cryosurgery in the treatment of giant
cell tumor. A long term follow-up study. Clin Orthop Relat Res.
176–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcove RC, Steth DS, Brien EW, Huvos AG
and Healey JH: Conservative surgery for giant cell tumors of the
sacrum. The role of cryosurgery as a supplement to curettage and
partial excision. Cancer. 74:1253–1260. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi W, Indelicato DJ, Reith J, Smith KB,
Morris CG, Scarborough MT, Gibbs CP Jr, Mendenhall WM and Zlotecki
RA: Radiotherapy in the management of giant cell tumor of bone. Am
J Clin Oncol. 36:505–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin PP, Guzel VB, Moura MF, Wallace S,
Benjamin RS, Weber KL, Morello FA Jr, Gokaslan ZL and Yasko AW:
Long-term follow-up of patients with giant cell tumor of the sacrum
treated with selective arterial embolization. Cancer. 95:1317–1325.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lackman RD, Khoury LD, Esmail A and
Donthineni-Rao R: The treatment of sacral giant-cell tumours by
serial arterial embolisation. J Bone Joint Surg Br. 84:873–877.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onishi H, Kaya M, Wada T, Sagoya S, Sasaki
M and Yamashita T: Giant cell tumor of the sacrum treatment with
selective arterial embolization. Int J Clin Oncol. 15:416–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balke M, Campanacci L, Gebert C, Picci P,
Gibbons M, Taylor R, Hogendoorm P, Kroep J, Wass J and Athanasou N:
Bisphosphonate treatment of aggressive primary, recurrent and
metastatic giant cell tumour of bone. BMC Cancer. 10:4622010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishisho T, Hanaoka N, Endo K, Takahashi M
and Yasui N: Locally administered zoledronic acid therapy for giant
cell tumor of bone. Orthopedics. 34:e312–e315. 2011.PubMed/NCBI
|
|
12
|
Tse LF, Wong KC, Kumta SM, Huang L, Chow
TC and Griffith JF: Bisphosphonates reduce local recurrence in
extremity giant cell tumor of bone: A case-control study. Bone.
42:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chawla S, Henshw R, Seeger L, Choy E, Blay
JY, Ferrari S, Krope J, Grimer R, Reichardt P, Rutkowski P, et al:
Safety and efficacy of denosumab for adults and skeletally mature
adolescents with giant cell tumour of bone: Interim analysis of an
open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raslin KA, Schwab JH, Mankin HJ,
Springfield DS and Hornicek FJ: Giant cell tumor of bone. J Am Acad
Orthop Surg. 21:118–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruggieri P, Mavrogenis AF, Ussia G,
Angelini A, Papagelopoulos PJ and Mercuri M: Recurrence after and
complications associated with adjuvant treatments for sacral giant
cell tumor. Clin Orthop Relat Res. 468:2954–2961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueda T, Morioka H, Nishida Y, Kakunaga S,
Tsuchiya H, Matsumoto Y, Asami Y, Inoue T and Yoneda T: Objective
tumor response to denosumab in patients with giant cell tumor of
bone: A multicenter phase II trial. Ann Oncol. 26:2149–2154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase II trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shinozaki T, Saito K, Kobayashi T,
Yanagawa T and Takagishi K: Tartrate-resistant acid phosphatase 5b
is a useful serum marker for diagnosis and recurrence detection of
giant cell tumor of bone. Open Orthop J. 6:392–399. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Branstetter DG, Nelson SD, Manivel JC,
Blay JY, Chawla S, Thomas DM, Jun S and Jacobs I: Denosumab induces
tumor reduction and bone formation in patients with giant-cell
tumor of bone. Clin Cancer Res. 18:4415–4424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau CP, Huang L, Wong KC and Kumta SM:
Comparison of the anti-tumor effect of denosumab and zoledronic
acid on the neoplastic stromal cells of giant cell tumor of bone.
Connect Tissue Res. 54:439–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baron R, Ferrari S and Russell RG:
Denosumab and bisphosphonates: Different mechanisms of action and
effects. Bone. 48:677–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zebaze RM, Libanati C, Austin M,
Ghasem-Zadeh A, Hanley DA, Zanchetta JR, Thomas T, Boutroy S,
Bogado CE, Bilezikian JP and Seeman E: Differing effects of
denosumab and alendronate on cortical and trabecular bone. Bone.
59:173–179. 2014. View Article : Google Scholar : PubMed/NCBI
|