Introduction
Xp11.2 translocation renal cell carcinoma (RCC)
involves fusion between the transcription factor binding to IGHM
enhancer 3 (TFE3) in chromosome Xp11.2 and various partners, and
was classified as a separate subset of RCCs by the World Health
Organization in 2004 (1). According
to a published article, 1.6% of adult RCCs involve Xp11.2
translocation and 15% of patients aged <45 years had
translocation RCC (2). Compared with
conventional RCC, this type of RCC is mostly diagnosed at an
advanced stage, as it exhibits an aggressive course (3–7).
However, a systemic therapy for metastatic adult Xp11.2
translocation RCC has not yet been established. We herein report a
case of metastatic Xp11.2 translocation RCC that was oncologically
controlled with cytoreductive nephrectomy (CN) and axitinib
therapy.
Case report
A 57-year-old woman presented to a local hospital
with fatigue and low back pain in October 2014. The laboratory
tests revealed high levels of aspartate aminotransferase, alanine
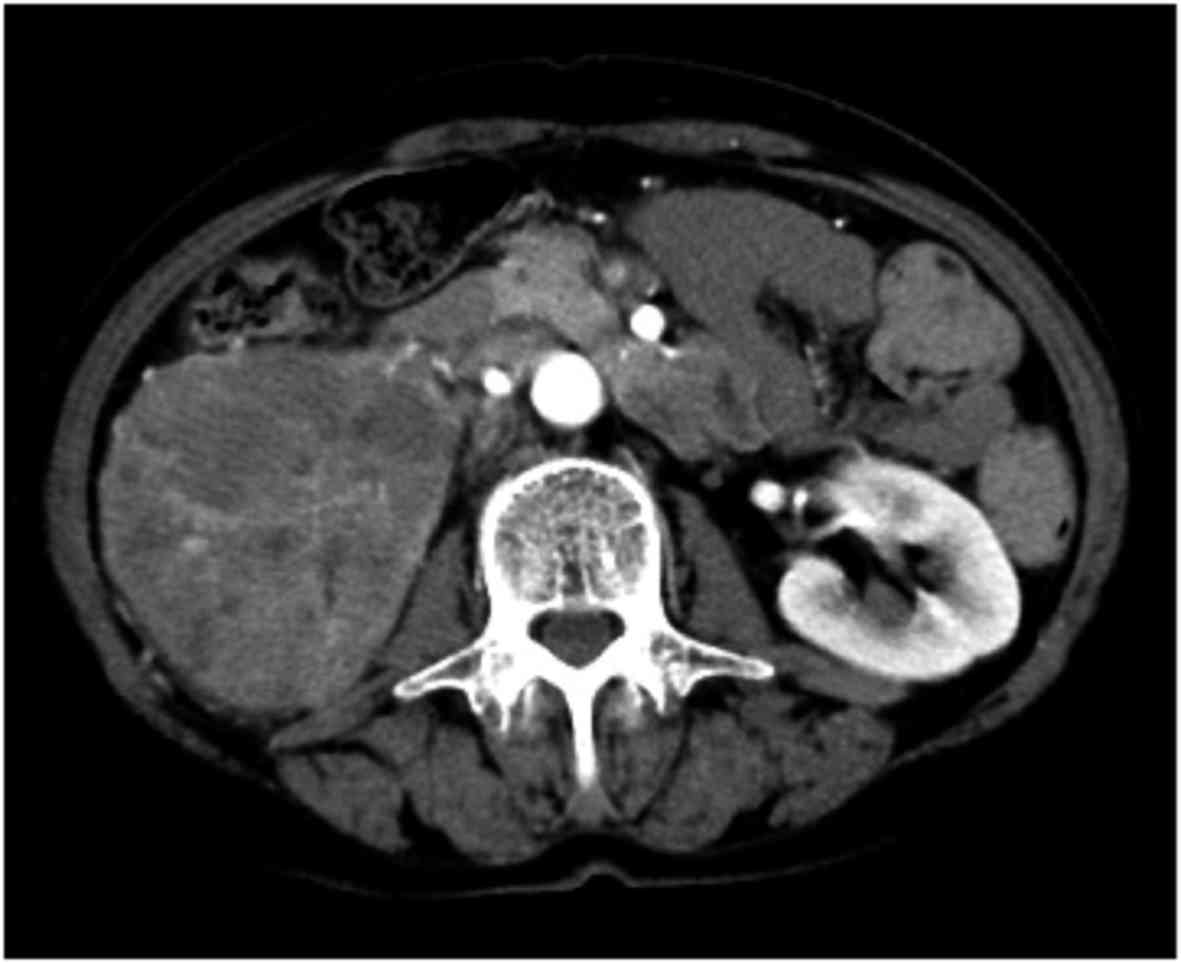
aminotransferase and lactate dehydrogenase. Ultrasonography and
computed tomography revealed a right renal tumor, 80 mm in
diameter, extending to the renal vein. Multiple lung nodules,
lymphadenopathy of the mediastinal lymph nodes and pulmonary hilar
lymph nodes were also identified (Fig.
1). The patient was diagnosed with RCC of clinical stage
T3aN0M1, with intermediate risk according to the Memorial
Sloan-Kettering Cancer Center risk classification (8). Although axitinib treatment was
administered for 5 months at the initial hospital, the status of
the primary and metastatic lesions remained unchanged. Thus, the
patient was referred to the Tokyo Women's Medical University
Hospital for further treatment in January 2015. CN was planned,
followed by targeted therapy. On macroscopic examination following
CN, almost the entire kidney was replaced by a yellowish brown
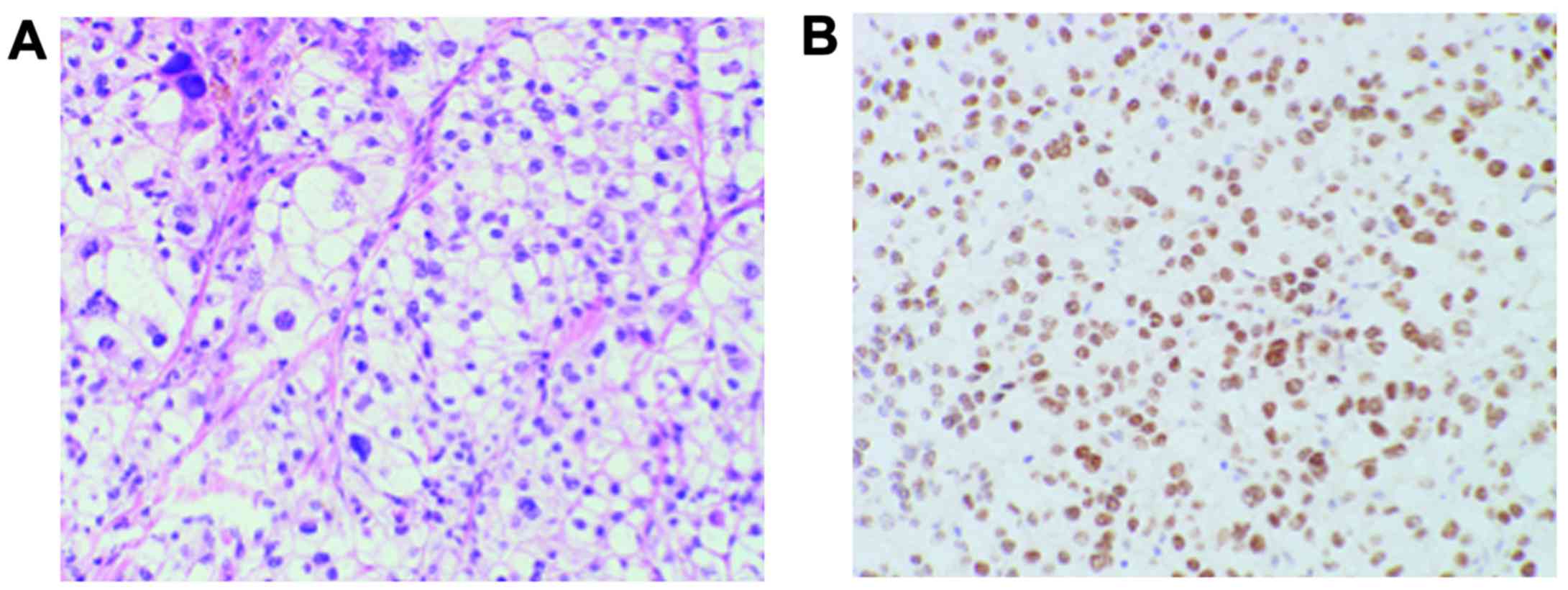
tumor >80 mm in diameter. Histological examination of
hematoxylin and eosin-stained sections revealed that the tumor was
composed of cells with a voluminous clear cytoplasm and pleomorphic
nuclei with prominent nucleoli (Fig.
2A). In addition, TFE3 immunostaining was positive (Fig. 2B). Thus, the pathological diagnosis
was Xp11.2 translocation RCC. Axitinib treatment was resumed 1
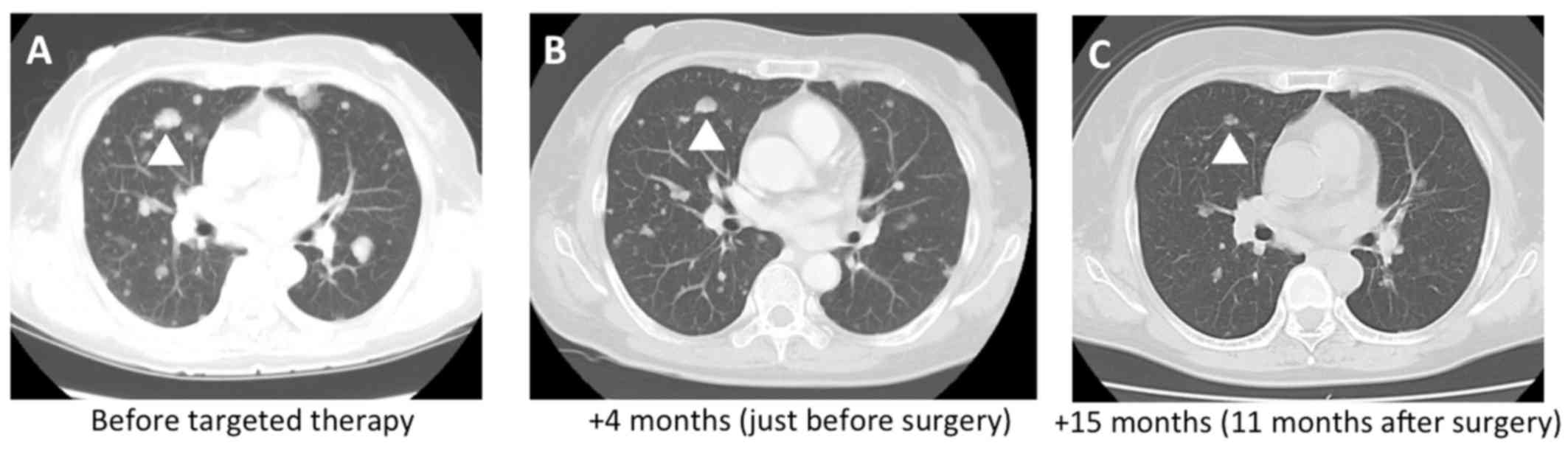
month after surgery. At 11 months after surgery, follow-up computed
tomography revealed that the size of the metastatic lung lesions
was decreased by 11%, despite the limited effectiveness of the
preoperative axitinib therapy (Fig.
3). Adverse events included grade 2 hypertension, grade 2
digestive symptoms and grade 2 hand-foot syndrome, assessed
according to the Common Terminology Criteria for Adverse Events,
version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
The patient maintained stable disease for >12 months after the
surgery and received the last ambulatory treatment in October
2016.
Discussion
We herein report a case of metastatic Xp11.2
translocation RCC that was oncologically controlled with CN and
axitinib therapy for >12 months. In addition, CN may have
enhanced the effectiveness of the axitinib therapy in this case.
Xp11.2 translocation RCC includes a translocation that activates
the MET protein owing to the TFE3 gene on the X chromosome or the
microphthalmia-associated transcription factor (MiTF) on chromosome
6 (6). Several reports investigated
the management of metastatic Xp11.2 translocation RCC by
immunotherapy (2,5), but the response to this type of therapy
was poor. Regarding targeted therapy, previous multicenter
retrospective studies of sunitinib reported a median
progression-free survival time of 7.1–8.2 months (9,10). One
pediatric case of metastatic Xp11.2 translocation RCC was
controlled with axitinib therapy (11); however, to the best of our knowledge,
reports of adult cases oncologically controlled with axitinib
therapy are not available in the literature. The benefit of CN was
evaluated by previous prospective randomized research studies in
the cytokine therapy era (12,13). By
contrast, the usefulness of CN in the new era of targeted therapy
has not been confirmed in a large randomized trial, although
several retrospective studies have demonstrated the effectiveness
of CN (14,15). Heng et al assessed the overall
survival benefit of CN in comparison with targeted therapy without
CN in metastatic RCC (mRCC) patients according to the International
Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria
(16); the authors demonstrated that
CN is beneficial in patients with synchronous mRCC treated with
targeted therapy, even in cases of non-clear cell histology. In
addition, they found that the majority of the patients benefited
from CN, except for those with ≥4 IMDC risk factors (17). In the present case, axitinib therapy
was commenced prior to nephrectomy at the initial hospital, despite
the presence of one IMDC risk factor; the time from diagnosis to
initial treatment was <1 year. Five months of axitinib therapy
did not reduce the size of the primary or metastatic lesions.
However, CN followed by axitinib therapy resulted in reduction of
the size of the lung and lymph node metastases, suggesting that CN
may enhance the effectiveness of axitinib therapy. However, whether
CN prolongs survival in the targeted era remains unclear and, if
so, it should be performed before or after targeted therapy. The
results of two ongoing prospective randomized trials, the Clinical
Trial to Assess the Importance of Nephrectomy (CARMENA; NCT0093033)
and the European Organization for Research and Treating Patients
with Metastatic Kidney Cancer trial (SURTIME; NCT01099423), are
expected.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
CN
|
cytoreductive nephrectomy
|
References
|
1
|
Armah HB and Parwani AV: Xp11.2
translocation renal cell carcinoma. Arch Pathol Lab Med.
134:124–129. 2010.PubMed/NCBI
|
|
2
|
Komai Y, Fujiwara M, Fujii Y, Mukai H,
Yonese J, Kawakami S, Yamamoto S, Migita T, Ishikawa Y, Kurata M,
et al: Adult Xp11 translocation renal cell carcinoma diagnosed by
cytogenetics and immunohistochemistry. Clin Cancer Res.
15:1170–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Argani P, Olgac S, Tickoo SK, Goldfischer
M, Moch H, Chan DY, Eble JN, Bonsib SM, Jimeno M, Lloreta J, et al:
Xp11 translocation renal cell carcinoma in adults: Expanded
clinical, pathologic, and genetic spectrum. Am J Surg Pathol.
31:1149–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rais-Bahrami S, Drabick JJ, De Marzo AM,
Hicks J, Ho C, Caroe AE and Argani P: Xp11 translocation renal cell
carcinoma: Delayed but massive and lethal metastases of a
chemotherapy-associated secondary malignancy. Urology.
70:178.e3–178.e6. 2007. View Article : Google Scholar
|
|
5
|
Armah HB and Parwani AV: Renal cell
carcinoma in a 33-year-old male with an unusual morphology and an
aggressive clinical course: Possible Xp11.2 translocation.
Pathology. 40:306–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsuda M, Davis IJ, Argani P, Shukla N,
McGill GG, Nagai M, Saito T, Laé M, Fisher DE and Ladanyi M: TFE3
fusions activate MET signaling by transcriptional up-regulation,
defining another class of tumors as candidates for therapeutic MET
inhibition. Cancer Res. 67:919–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Camparo P, Vasiliu V, Molinie V, Couturier
J, Dykema KJ, Petillo D, Furge KA, Comperat EM, Lae M, Bouvier R,
et al: Renal translocation carcinomas: Clinicopathologic,
immunohistochemical, and gene expression profiling analysis of 31
cases with a review of the literature. Am J Surg Pathol.
32:656–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choueiri TK, Lim ZD, Hirsch MS, Tamboli P,
Jonasch E, McDermott DF, Dal Cin P, Corn P, Vaishampayan U, Heng
DY, et al: Vascular endothelial growth factor-targeted therapy for
the treatment of adult metastatic Xp11.2 translocation renal cell
carcinoma. Cancer. 116:5219–5225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malouf GG, Camparo P, Oudard S,
Schleiermacher G, Theodore C, Rustine A, Dutcher J, Billemont B,
Rixe O, Bompas E, et al: Targeted agents in metastatic Xp11
translocation/TFE3 gene fusion renal cell carcinoma (RCC): A report
from the Juvenile RCC Network. Ann Oncol. 21:1834–1838. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sudour-Bonnange H, Leroy X, Chauvet MP,
Classe M, Robin PM and Leblond P: Cutaneous metastases during an
aggressive course of Xp11.2 translocation renal cell carcinoma in a
teenager. Pediatr Blood Cancer. 61:1698–1700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flanigan RC, Salmon SE, Blumenstein BA,
Bearman SI, Roy V, McGrath PC, Caton JR Jr, Munshi N and Crawford
ED: Nephrectomy followed by interferon alfa-2b compared with
interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J
Med. 345:1655–1659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mickisch GH, Garin A, van Poppel H, de
Prijck L and Sylvester R: European Organisation for Research and
Treatment of Cancer (EORTC) Genitourinary Group: Radical
nephrectomy plus interferon-alfa-based immunotherapy compared with
interferon alfa alone in metastatic renal-cell carcinoma: A
randomised trial. Lancet. 358:966–970. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choueiri TK, Xie W, Kollmannsberger C,
North S, Knox JJ, Lampard JG, McDermott DF, Rini BI and Heng DY:
The impact of cytoreductive nephrectomy on survival of patients
with metastatic renal cell carcinoma receiving vascular endothelial
growth factor targeted therapy. J Urol. 185:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conti SL, Thomas IC, Hagedorn JC, Chung
BI, Chertow GM, Wagner TH, Brooks JD, Srinivas S and Leppert JT:
Utilization of cytoreductive nephrectomy and patient survival in
the targeted therapy era. Int J Cancer. 134:2245–2252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heng DY, Wells JC, Rini BI, Beuselinck B,
Lee JL, Knox JJ, Bjarnason GA, Pal SK, Kollmannsberger CK, Yuasa T,
et al: Cytoreductive nephrectomy in patients with synchronous
metastases from renal cell carcinoma: Results from the
International Metastatic Renal Cell Carcinoma Database Consortium.
Eur Urol. 66:704–710. 2014. View Article : Google Scholar : PubMed/NCBI
|