Introduction
An estimated 1.8 million new lung cancer cases
occurred in 2012, accounting for ~13% of total cancer diagnoses,
making lung cancer the most frequently diagnosed malignancy and
leading cause of cancer-related mortality among men worldwide and
among women in more developed countries (1). Small-cell lung cancer (SCLC) accounts
for only 13% of all lung cancers and has a very poor prognosis,
with ~5% of patients with extensive-disease (ED) SCLC surviving for
2 years (2). Although paraneoplastic
syndromes are fairly common in SCLC (3), it is estimated that <5% of patients
with SCLC will develop a clinically significant paraneoplastic
neurological disorder (PND) (4).
Paraneoplastic limbic encephalitis (PLE) is a rare disorder
infrequently accompanying cancer, coexisting with SCLC in ~50% of
the cases (5). It was suggested that
the prognosis of cancer patients with accompanying paraneoplastic
syndromes may be better compared with that of patients with the
same cancer but without associated paraneoplasia (6–8).
Over the last few years, a major advancement in
understanding SCLC biology has been made (9), which may finally lead to the
introduction of new drugs and improvement of the treatment
efficacy. Preclinical data demonstrated that the addition of
valproic acid (VPA), a potent histone deacetylase inhibitor, to
standard chemotherapy regimens in SCLC, may improve patient outcome
(10,11).
We herein report on the case of a patient with ED
SCLC who developed PLE during the course of his disease, with
symptoms of PLE typically preceding the diagnosis of SCLC. The
diagnostic process of the SCLC and the PLE in this patient were
also described and the potential synergistic effect of the VPA and
chemotherapy in the treatment of the SCLC was investigated.
Case report
A 50-year-old man with no medical history was
admitted to the community hospital in July, 2007 with generalized
seizures. The patient was a heavy cigarette smoker (~70 pack-years)
and had a history of alcohol abuse. The family history was
insignificant. On admission, the computed tomography (CT) scan of
the brain revealed no abnormalities, but the chest X-ray showed
enlarged right hilar nodes. The blood count, liver and renal
function tests were within normal limits. Treatment with VPA and
dexamethasone was initiated. Two days later, the patient developed
symptoms of acute psychosis and was referred to the Department of
Psychiatry at the University Hospital in Krakow. Finally, due to
fever (temperature up to 39.6°C) and suspicion of central nervous
system infection, the patient was transferred to the Department of
Infectious Diseases. On physical examination, the patient was
febrile and conscious; however, he was somnolent with significant
short-term memory loss and capable of responding only to basic
questions. No focal neurological deficits or neck rigidity were
present. The results of the cerebrospinal fluid (CSF) examination
(colour, yellowish; clarity, muddy; cytosis, 13 cells/µl;
protein content, 0.49 g/l; glucose, 2.3 mmol/l; chloride, 127
mmol/l) were compatible with the diagnosis of lymphocytic
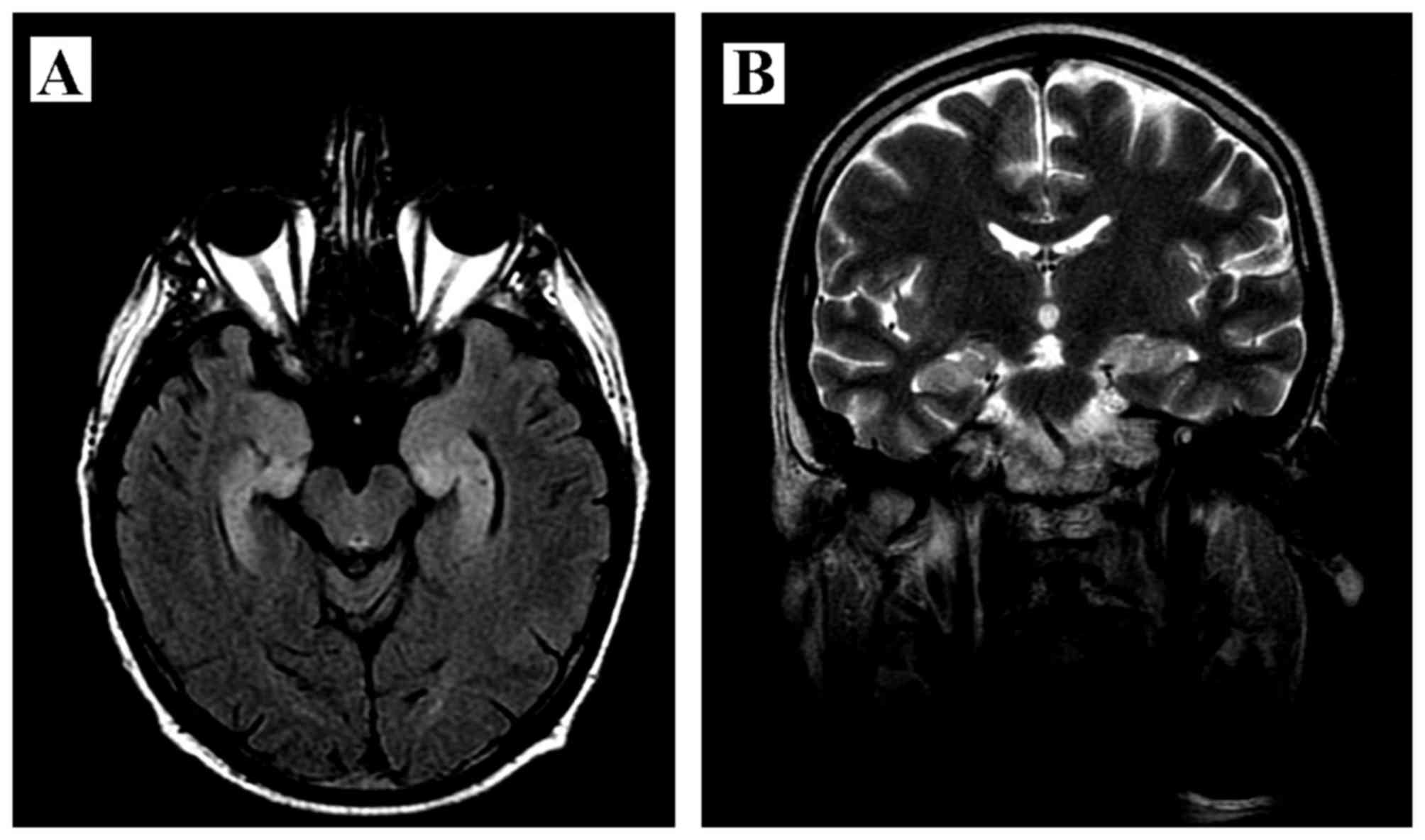
meningitis. The magnetic resonance imaging (MRI) of the brain
revealed a mild enlargement and hyperintensity of the hippocampal
gyri bilaterally, with narrowed temporal horns of the lateral
ventricles (Fig. 1). The CSF
cultures and the serological test for Lyme disease were both
negative. The chest and abdominal CT scan revealed a 10×5-cm
neoplastic mass in the mediastinum, enlarged right hilar,
subcarinal, paratracheal and left hilar lymph nodes, as well as a
right hydrothorax. The biopsy of the enlarged right hilar nodes was
positive for SCLC.
After a course of acyclovir and antibiotics
(ceftriaxone, rifampicin, isoniazid, imipenem/cilastatin and
linezolid), the patient was subsequently referred to the Department
of Oncology for further treatment of the ED SCLC. First-line
palliative chemotherapy with cisplatin and etoposide was decided.
At that moment, based on the combination of the clinical picture
(seizures, personality changes, short-term memory loss,
pathological voracity), the MRI results and the presence of SCLC,
PLE accompanying the SCLC was suspected. PLE was subsequently
confirmed by the presence of anti-Hu autoantibodies in the
patient's serum. Treatment with corticosteroids was continued, with
the aim of suppressing the immunological system response and
reducing the symptoms of the paraneoplastic syndrome. After 6
cycles of chemotherapy, having achieved a partial response to the
treatment, the patient underwent prophylactic cranial irradiation
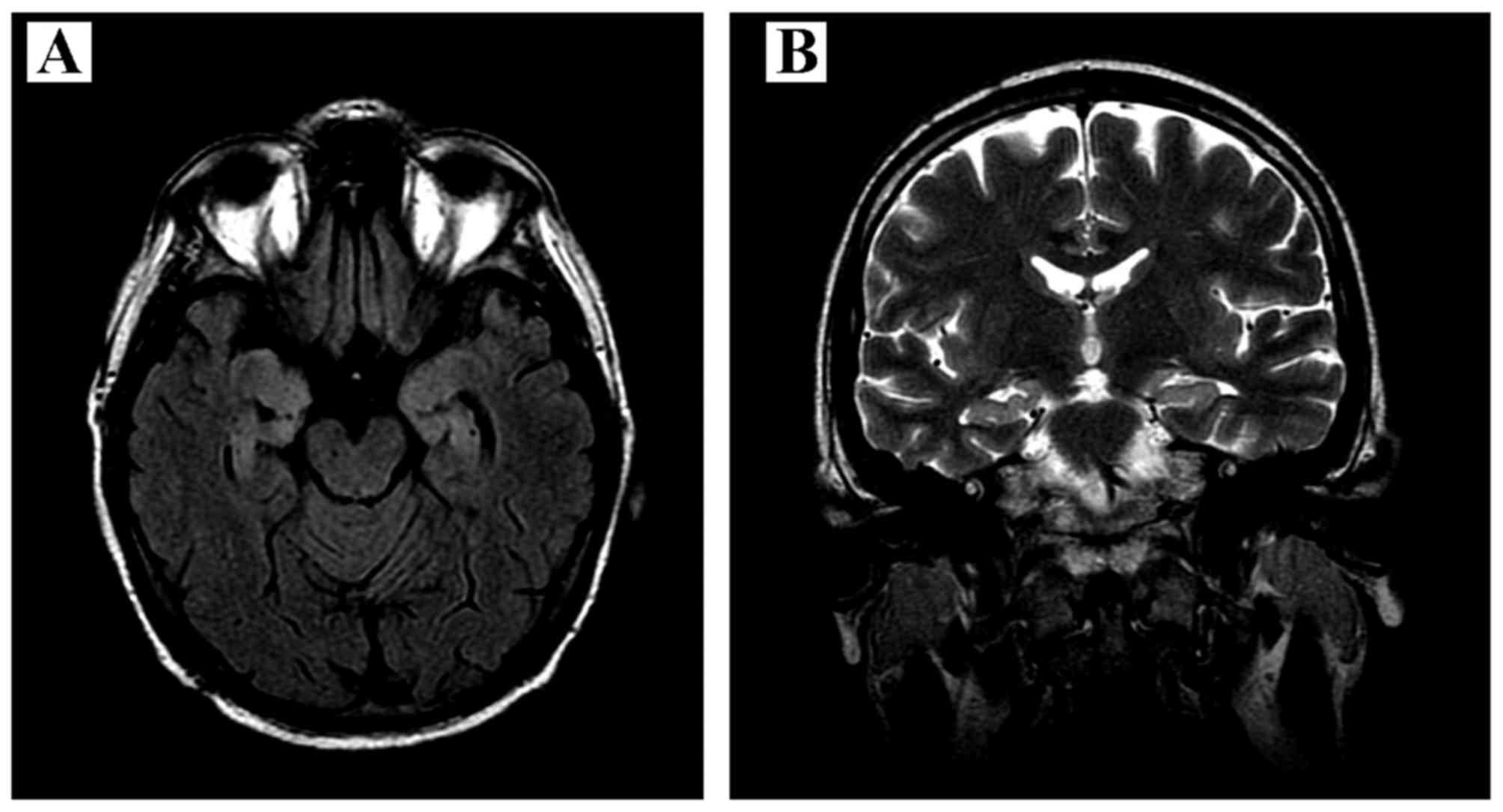
(30 Gy in 15 fractions). The subsequent brain MRI showed less
prominent hyperintensity on T2-weighted images in the hippocampal
gyri bilaterally, with signs of limbic system atrophy (Fig. 2). There was no amelioration of the
brain functions after completion of these therapies, except for a
mild improvement of the patient's memory.
Five months later, a control CT scan revealed tumor
progression and the patient received 6 cycles of second-line
chemotherapy with cyclophosphamide, doxorubicin and vincristine
(CAV regimen). At the next progression, the patient was treated
with palliative radiotherapy for the tumor in the chest and
cyclophosphamide monotherapy. Finally, the patient succumbed to the
disease 25 months after the diagnosis of the ED SCLC.
Discussion
The clinical diagnosis of PLE may be challenging, as
its typical symptoms (subacute cognitive dysfunction with severe
memory impairment, seizures and psychiatric features including
depression, anxiety and hallucinations) may be similar to those
observed in other cancer-related complications, metabolic and toxic
encephalopathies, infections (particularly herpes simplex
encephalitis) and to the side effects of cancer therapy (5,12–14). In
addition, the presenting symptoms may differ from those considered
typical of the syndrome and the majority of the patients are not
known to have cancer (5). However,
fulfilling all the following criteria for PLE may facilitate its
diagnosis: i) A compatible clinical picture; ii) an interval of
<4 years between the onset of neurological symptoms and cancer
diagnosis; iii) exclusion of other cancer-related complications
(metastasis, infection, metabolic and nutritional deficits,
cerebrovascular disorder or side effects of therapy) that may cause
symptoms of limbic dysfunction; iv) at least one of the following:
CSF with inflammatory changes, MRI showing unilateral or bilateral
temporal lobe abnormalities on T2-weighted images or atrophy on
T1-weighted images, and an electroencephalogram showing slow- or
sharp-wave activity in one or both temporal lobes (5).
MRI examination of the brain and the spinal cord are
required to exclude other causes of the neurological symptoms,
including cancer metastases. The typical initial MRI findings in
PLE [common FLAIR hyperintensity in medial temporal lobes (15)] may be similar to the ones observed in
other forms of encephalitis of the limbic system, particularly
during the early stages of herpes simplex encephalitis. However,
patients with herpes simplex encephalitis usually display distinct
characteristics: Prominent edema and mass effect involving one or
both inferior-medial temporal lobes, the inferior frontal lobes and
the cingulate gyrus, gyral enhancement in the majority of cases
and, frequently, signs of hemorrhage (5,16).
The CSF examination in a patient with PLE is often
abnormal, with mild to moderate pleocytosis, increased protein
content, intrathecal synthesis of IgG and oligoclonal bands,
suggesting the presence of an inflammatory or immune-mediated
disorder (4,5).
The majority of the paraneoplastic syndromes
involving the nervous system are currently considered to be
immune-mediated (17). Ectopic
expression by a tumor of the antigens normally present in the
neurons (referred to as onconeural antigens) induces an immune
system response with production of specific antibodies and
activation of T-cells that, after crossing the blood-brain barrier,
react with the onconeural antigens and produce specific
neurological symptoms (17).
Therefore, the majority of patients with paraneoplastic syndromes
involving the nervous system have detectable antineuronal
antibodies in their serum and/or the CSF (4,5) and
their presence may facilitate the diagnosis of the paraneoplastic
syndrome and allow early detection of the associated tumor
(12). Anti-Hu antibodies are
typically found in SCLC, neuroblastoma and prostate cancer
(17). SCLC patients with anti-Hu
antibodies have a lower probability of improvement in PLE symptoms
compared with patients without anti-Hu antibodies (12), and only a minority of these patients
responds to treatment with corticosteroids, intravenous
immunoglobulins or plasma exchange (18).
With the currently available therapy, the median
survival of patients with ED SCLC is 6–12 months, with an overall
survival of <5% after 2 years (2,10). The
prolonged survival in our patient may result from several factors.
First, the prognosis of cancer patients with a paraneoplastic
syndrome appears to be better compared with that of patients with
the same malignancy but without the associated paraneoplastic
disorder (6–8). It is considered that the immune
response triggered by onconeural antigens not only injures a
specific part of the nervous system and is responsible for the
clinical presentation of the paraneoplastic syndrome, but may also
control tumor growth (8). Second,
the chronic use of VPA may have improved the outcome in this
patient. VPA is a potent histone deacetylase inhibitor and may
affect the dynamic structure of chromatin and the transcriptional
processes leading to the modulation of pathways involved in cell
cycle and apoptosis (10,11,19).
Moreover, preclinical data demonstrated that the combination of VPA
and standard chemotherapy regimens in SCLC may exert a synergistic
antitumor effect (10,11).
In conclusion, the case presented herein underlines
the need for precise evaluation of all cancer patients presenting
with symptoms of a paraneoplastic disorder. These symptoms usually
precede the diagnosis of the cancer itself and may facilitate the
diagnosis of the underlying malignancy. Moreover, this case
suggests that the efficacy of the standard chemotherapy regimens in
SCLC may be further improved by additional agents, such as VPA,
which may reverse epigenetic changes in cancer cells.
Acknowledgements
The authors would like to thank Joanna Gołąb, the
language editor, for her help in the preparation of this
manuscript.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puglisi M, Dolly S, Faria A, Myerson JS,
Popat S and O'Brien ME: Treatment options for small cell lung
cancer-do we have more choice? Br J Cancer. 102:629–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amini A, Byers LA, Welsh JW and Komaki RU:
Progress in the management of limited-stage small cell lung cancer.
Cancer. 120:790–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bataller L and Dalmau J: Paraneoplastic
neurologic syndromes. Neurol Clin. 21:221–247, ix. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gultekin SH, Rosenfeld MR, Voltz R, Eichen
J, Posner JB and Dalmau J: Paraneoplastic limbic encephalitis:
Neurological symptoms, immunological findings and tumour
association in 50 patients. Brain. 123:1481–1494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altman AJ and Baehner RL: Favorable
prognosis for survival in children with coincident opso-myoclonus
and neuroblastoma. Cancer. 37:846–852. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maddison P, Newsom-Davis J, Mills KR and
Souhami RL: Favourable prognosis in Lambert-Eaton myasthenic
syndrome and small-cell lung carcinoma. Lancet. 353:117–118. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graus F, Dalmou J, Reñé R, Tora M, Malats
N, Verschuuren JJ, Cardenal F, Viñolas N, del Muro J Garcia, Vadell
C, et al: Anti-Hu antibodies in patients with small-cell lung
cancer: Association with complete response to therapy and improved
survival. J Clin Oncol. 15:2866–2872. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altan M and Chiang AC: Management of small
cell lung cancer: Progress and Updates. Cancer J. 21:425–433. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hubaux R, Vandermeers F, Crisanti MC,
Kapoor V, Burny A, Mascaux C, Albelda SM and Willems L: Preclinical
evidence for a beneficial impact of valproate on the response of
small cell lung cancer to first-line chemotherapy. Eur J Cancer.
46:1724–1734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hubaux R, Vandermeers F, Cosse JP,
Crisanti C, Kapoor V, Albelda S, Mascaux C, Delvenne P, Hubert P
and Willems L: Valproic acid improves second-line regimen of small
cell lung carcinoma in preclinical models. ERJ Open Res.
1:00028–2015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alamowitch S, Graus F, Uchuya M, Reñé R,
Bescansa E and Delattre JY: Limbic encephalitis and small cell lung
cancer. Clinical and immunological features. Brain. 120:923–928.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newman NJ, Bell IR and McKee AC:
Paraneoplastic limbic encephalitis: Neuropsychiatric presentation.
Biol Psychiatry. 27:529–542. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bakheit AM, Kennedy PG and Behan PO:
Paraneoplastic limbic encephalitis: Clinico-pathological
correlations. J Neurol Neurosurg Psychiatry. 53:1084–1088. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalmau J and Rosenfeld MR: Paraneoplastic
syndromes of the CNS. Lancet Neurol. 7:327–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demaerel P, Wilms G, Robberecht W,
Johannik K, Van Hecke P, Carton H and Baert AL: MRI of herpes
simplex encephalitis. Neuroradiology. 34:490–493. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Darnell RB and Posner JB: Paraneoplastic
syndromes involving the nervous system. N Engl J Med.
349:1543–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graus F, Keime-Guibert F, Reñe R, Benyahia
B, Ribalta T, Ascaso C, Escaramis G and Delattre JY:
Anti-Hu-associated paraneoplastic encephalomyelitis: Analysis of
200 patients. Brain. 124:1138–1148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duenas-Gonzalez A, Candelaria M,
Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E and
Herrera LA: Valproic acid as epigenetic cancer drug: Preclinical,
clinical and transcriptional effects on solid tumors. Cancer Treat
Rev. 34:206–222. 2008. View Article : Google Scholar : PubMed/NCBI
|