Introduction
Gastric cancer is the fifth most common malignancy,
and has the third highest rate of cancer-specific mortality
worldwide (1). In excess of 70% of
the cases worldwide occur in developing countries, and over half
occur in Eastern Asia, predominantly in China (1). A large number of patients are diagnosed
at an inoperable stage of the disease, or experience disease
recurrence, indicating a poor outcome (2). The 5-year survival rate of advanced
gastric cancer (AGC) was <1%, and the median overall survival
(OS) rate was determined to be <1 year (3). Palliative chemotherapy is an important
treatment option for patients with AGC, since it improves survival
and the quality of life in patients with a good performance status
(4).
Systemic chemotherapy, with a combination of
fluoropyrimidine and platinum, is now regarded as the standard
treatment for these patients (4).
Over the course of the last few years, several single or combined
chemotherapy regimens containing S-1, capecitabine, platinum agents
(cisplatin and oxaliplatin), taxanes (paclitaxel and docetaxel) and
irinotecan have demonstrated potent effects for gastric cancer
(5–11). In addition, a trastuzumab for gastric
cancer (ToGA) study reported a clinical benefit of trastuzumab
treatment for patients with human epidermal growth factor receptor
2 (HER2)-positive gastric cancer (12).
However, the efficacy of first-line treatment is
modest, and the majority of patients either do not respond to
therapy, or eventually experience disease progression. Patients
with refractory gastric cancer often receive salvage chemotherapy
clinically. The results of two randomized controlled trials (RCTs)
were published, revealing an OS benefit from treatments with
irinotecan or docetaxel compared with best supportive care (BSC)
alone in patients for whom one or two prior treatments had failed
(13,14). Recently, Kim et al (15) reported a meta-analysis in which a
significant reduction in the risk of death was observed in patients
receiving salvage chemotherapy compared with supportive cancer
treatment. This provided evidence for implementing second-line
chemotherapy in patients with AGC. Based on the variety of
patients, making the strategy for optimizing the regimens and
arranging the order of different regimens for individual patients
occasionally remain difficult for oncologists.
In the present study, the efficacy and safety of
first- and second-line chemotherapy in AGC patients was evaluated
with a retrospective analysis, the aim being to investigate the
best strategy for choosing chemotherapy regimens and to identify
whether increasing the number of treatment cycles will benefit
patients.
Patients and methods
Patients
Patients with advanced or recurrent gastric cancer,
who were treated with at least two chemotherapy regimens between
May 2006 and July 2014 at Zhejiang Cancer Hospital (Hangzhou,
China), were retrospectively investigated. Patients with
histologically confirmed advanced or recurrent gastric cancer were
retrospectively investigated. Chemotherapy regimens were
unrestricted. Furthermore, the eligibility criteria included at
least one measurable lesion of ≥1 cm in the longest diameter, or a
lymphonodus of ≥1.5 cm in the shortest diameter. The present study
was approved by the Ethics Committee and Institutional Review Board
of Zhejiang Cancer Hospital (Hangzhou, China), and was conducted in
compliance with the Declaration of Helsinki. Patients were followed
up until death, or until December 31, 2014 if the patients were
alive at that time.
Treatments
Chemotherapy regimens were unrestricted in the
present study. Systemic chemotherapy was either mono- or
combination chemotherapy, which demonstrated efficacy in gastric
cancer, including S1, capecitabine (Xeloda®), CPT-11
(irinotecan), SP, S-1 plus oxaliplatin (SOX), capecitabine plus
oxaliplatin (XELOX), capecitabine plus docetaxel (XT), folinic
acid, fluorouracil and oxaliplatin (FOLFOX), folinic acid,
fluorouracil and irinotecan (FOLFIRI), docetaxel and cisplatin
(DP), docetaxel and S-1 (DS), docetaxel plus capecitabine (DX),
docetaxel, cisplatin and 5-fluorouracil DCF, and so forth. The
doses of chemotherapy, and their adjustments, were determined
according to the specific situation of each patient. After
progression of the disease had occurred, it usually became
necessary to switch to another chemotherapy regimen, or to stop
chemotherapy and continue with the best support treatment.
Adverse effects
Toxicity was measured using the National Cancer
Institute-Common Toxicity Criteria, version 2.0 toxicity scales
(16). Grade 3 to 4 toxicity was
recorded according to the medical records.
Assessment and statistics
The response was evaluated every two cycles of
treatment using the Response Evaluation Criteria in Solid Tumors
(17). Survival time was analyzed
using the Kaplan-Meier software of SPSS, version 15.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant value.
Results
Patient characteristics and clinical
data
A total of 248 patients with advanced or recurrent
gastric cancer who had received at least two chemotherapy regimens
were reviewed. Of those patients, 90 were excluded due to
incomplete follow-up data and an inability to perform a therapeutic
efficacy evaluation. This resulted in a total of 158 patients who
were suitable for a final evaluation.
The median age of the patients was 57 years and the
Karnofsky Performance Status (KPS) score was ≥80. A detailed
description of patient characteristics is shown in Table I. Treatment conditions of the
patients are shown in Table II, and
the specific regimens used in first- and second-line chemotherapies
are shown in Table III.
 | Table I.Baseline characteristics of patients
and related overall survival (OS) (n=158). |
Table I.
Baseline characteristics of patients
and related overall survival (OS) (n=158).
| Character | n (%) | mOS (days) | P-value |
|---|
| Gender |
|
| 0.469 |
| Male | 104 (65.8) | 335 |
|
|
Female | 54 (34.2) | 375 |
|
| Age |
|
| – |
|
Median | 57 | – |
|
|
Range | 24–76 |
|
|
| ≤60
years | 63 (39.9) | 351 | 0.793 |
| >60
years | 95 (60.1) | 364 |
|
| Radical
operation |
|
| 0.980 |
| Yes | 44 (27.8) | 333 |
|
| No | 114 (72.2) | 358 |
|
| Pathology |
|
| 0.687 |
| Well
differentiated | 1 (0.6) | 545 |
|
|
Moderately differentiated | 17 (10.8) | 372 |
|
| Poorly
differentiated | 114 (72.2) | 358 |
|
|
Differentiation unknown | 26 (16.4) | 320 |
|
| Primary tumor
site |
|
| 0.016 |
|
Esophagogastric junction | 23 (14.6) | 464 |
|
| Body of
stomach | 68 (43.0) | 329 |
|
| Gastric
antrum | 45 (28.5) | 405 |
|
| Diffuse
gastric lesions | 18 (11.4) | 356 |
|
|
Unkown | 4 (2.5) | 251 |
|
| Metastasis sites |
|
| 0.005 |
| Lymphatic
metastasis | 38 (24.0) | 412 |
|
|
Hematogenous metastasis | 26 (16.5) | 355 |
|
|
Peritoneal metastasis | 30 (19.0) | 392 |
|
| Mixture
metastasis | 64 (40.5) | 304 |
|
 | Table II.Treatment conditions of patients and
related overall survival (OS) (n=158). |
Table II.
Treatment conditions of patients and
related overall survival (OS) (n=158).
| Character | n (%) | mOS (days) | P-value |
|---|
| Lines of
chemotherapy |
|
| <0.001 |
| ≤2 | 103 (65.2) | 316 |
|
|
>2 | 55 (34.8) | 487 |
|
| First-line
chemotherapy |
|
| 0.647 |
|
Regimen |
|
|
|
|
Monochemotherapy | 9 (5.7) | 364 |
|
|
Doublet
chemotherapy | 134 (84.8) | 356 |
|
|
Triple
chemotherapy | 15 (9.5) | 321 |
|
|
Regimen containing
oxaliplatin | 76 (48.1) | 356 | 0.548 |
|
Regimen containing
taxanes | 41 (25.9) | 314 |
|
|
Regimen containing
Irinotecan and others | 41 (25.9) | 392 |
|
| Cycles (mean, 5;
range, 1–12) |
|
| <0.001 |
| 1–2
cycles | 28 (17.7) | 219 |
|
| 3–4
cycles | 38 (24.0) | 327 |
|
| 5–6
cycles | 60 (38.0) | 405 |
|
| >6
cycles | 32 (20.3) | 403 |
|
| Short-term
efficacy |
|
| <0.001 |
| CR + PR
+ SD | 112 (70.9) | 403 |
|
| PD | 46 (29.1) | 228 |
|
| Second-line
chemotherapy |
|
| 0.081 |
|
Regimen |
|
|
|
|
Monochemotherapy | 35 (22.1) | 313 |
|
|
Doublet
chemotherapy | 114 (72.2) | 371 |
|
|
Triple
chemotherapy | 9 (5.7) | 372 |
|
|
Regimen containing
oxaliplatin | 24 (15.2) | 314 | 0.544 |
|
Regimen containing
taxanes | 53 (33.5) | 378 |
|
|
Regimen containing
Irinotecan and others | 50 (31.6) | 364 |
|
|
Others | 31 (19.6) | 329 |
|
| Cycles (mean, 3;
range, 1–12) |
|
| <0.001 |
| 1–2
cycles | 65 (41.2) | 292 |
|
| 3–4
cycles | 50 (31.6) | 378 |
|
| 5–6
cycles | 28 (17.7) | 478 |
|
| >6
cycles | 15 (9.5) | 525 |
|
| Short-term
efficacy |
|
| <0.001 |
| CR (0)
+ PR + SD | 71 (44.9) | 421 |
|
| PD | 87 (55.1) | 301 |
|
 | Table III.Specific regimens used in first-line
and second-line chemotherapy. |
Table III.
Specific regimens used in first-line
and second-line chemotherapy.
| A, First-line
chemotherapy |
|---|
|
|---|
| Chemotherapy
regimen Regimen group | Specific
regimen | n (%) |
|---|
|
Oxaliplatin-containing regimen (n=76) | SOX | 45 |
|
| XELOX | 18 |
|
| FOLFOX | 13 |
| Taxane-containing
regimen (n=41) | TX/DX | 20 |
|
| TP/DP | 5 |
|
| TCF/DCF | 13 |
|
| TS/DS | 3 |
|
Irinotecan-containing regimen and others
(n=41) | FOLFIRI | 2 |
|
| Irinotecan | 1 |
|
|
Xeloda®/S1/XP/SP/ECF/FP | 38 |
|
| B, Second-line
chemotherapy |
|
| Chemotherapy
regimen Regimen group | Specific
regimen | n (%) |
|
|
Oxaliplatin-containing regimen (n=24) | SOX | 18 |
|
| XELOX | 3 |
|
| FOLFOX | 3 |
| Taxane-containing
regimen (n=53) | TX/DX | 12 |
|
| TP/DP | 12 |
|
| TCF/DCF | 14 |
|
| TS/DS | 9 |
|
| T/D | 6 |
|
Irinotecan-containing regimen (n=50) | FOLFIRI | 27 |
|
| Irinotecan | 15 |
|
| IP | 5 |
|
| IS | 3 |
| Others (n=31) |
Xeloda®/S1/XP/SP/ECF/FP | 31 |
Survival analysis
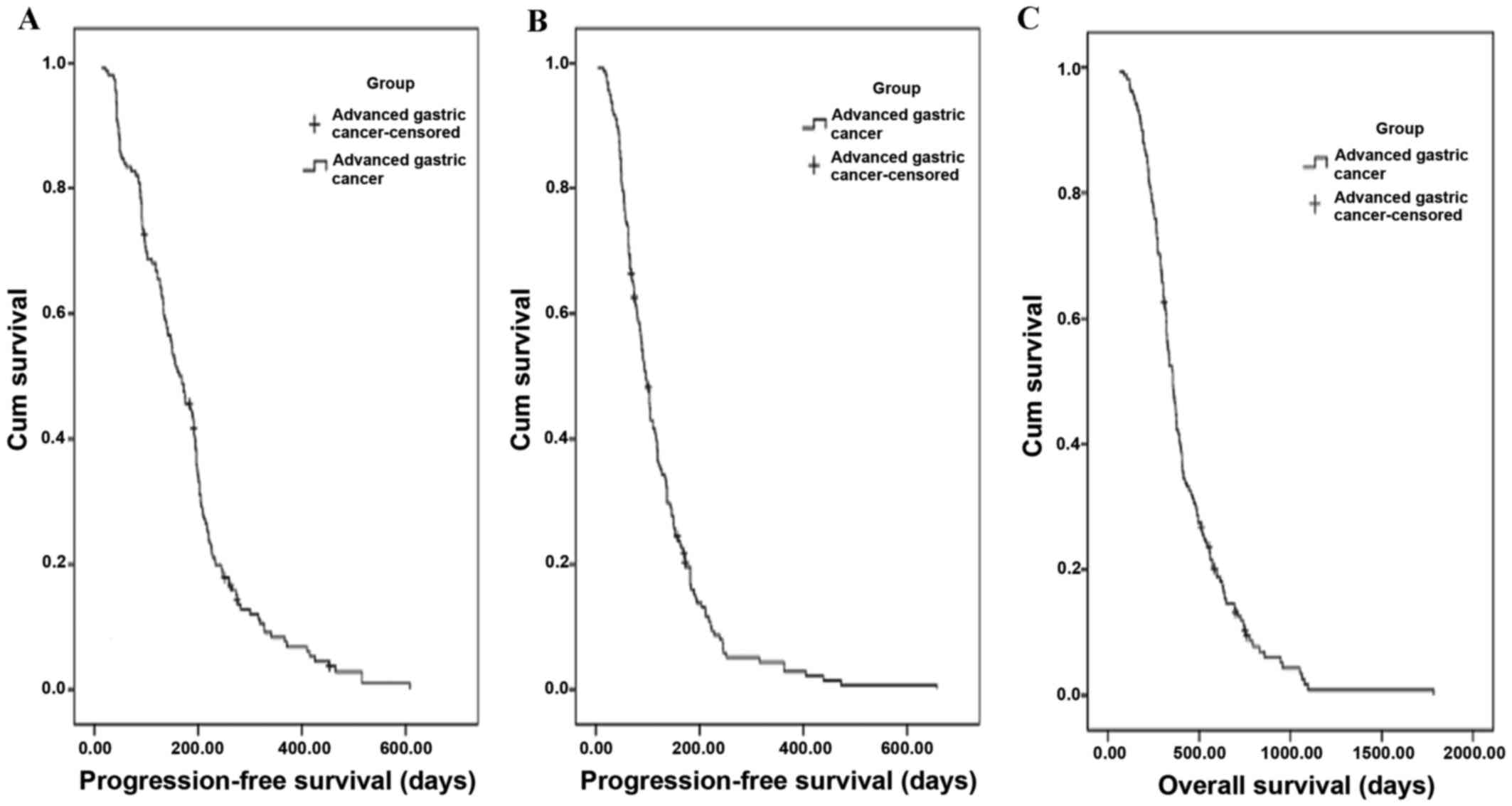
The Kaplan-Meier distribution curves of
progression-free survival (PFS) are shown in Fig. 1A and B for first- and second-line
chemotherapy, respectively. The PFS was 168 days [95% confidence
interval (CI), 140.6–195.4 days] in first-line chemotherapy, and 96
days (95% CI, 84.0–108.0 days) in second-line chemotherapy.
The Kaplan-Meier distribution of OS is shown in
Fig. 1C. The median OS was 356 days
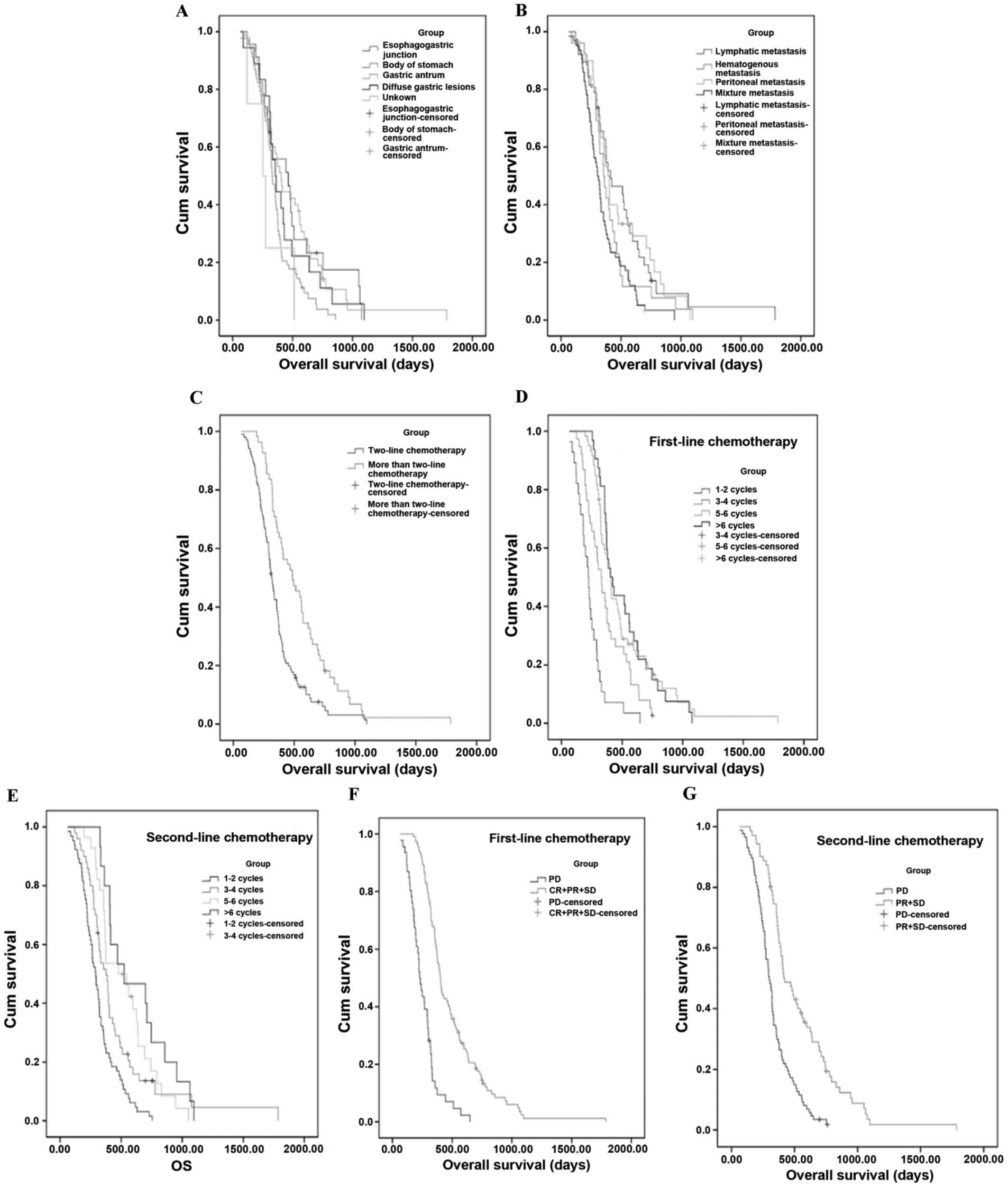
(95% CI, 323.7–388.4 days). Further subgroup analyses revealed that
OS was significantly longer for the following groups:
Esophagogastric junction tumor site (P=0.016; Fig. 2A) and lymphatic metastasis (P=0.005;
Fig. 2B). The histological factor
appeared to correlate with OS, of which patients with
well-differentiated tumors had the best OS, patients with poorly
differentiated tumors performed more poorly, and patients of
‘differentiation unknown’ status had the worst OS; however, the
difference was not significant (P=0.687). Other factors, including
gender, age, and whether a radical operation was received, had no
clear correlation with survival (Table
I). Several of the treatment conditions were significantly
associated with longer OS, as follows: Treated with >2 lines of
chemotherapy (P<0.001; Fig. 2C),
increased number of cycles of first-(P<0.001; Fig. 2D) and second-(P<0.001; Fig. 2E) line of chemotherapy, and good
short-term efficacy of first- (P<0.001; Fig. 2F) and second- (P<0.001; Fig. 2G) line of chemotherapy. Treatment
with more drugs in combination chemotherapy resulted in longer OS,
although the difference was not significant. Differences between
chemotherapy regimens had no obvious correlation with survival.
Safety
According to medical records, the toxicities were,
in the main, well tolerated. Grade 3 or 4 adverse events included
neutropenia (0.6% in the first-line chemotherapy in a triple
regimen, and 0.6% in the second-line chemotherapy) and
gastrointestinal reactions (0.6%). In addition, one patient
developed grade 2 weight loss on receiving second-line
chemotherapy. No neutropenic fever or treatment-associated
mortalities were documented.
Discussion
Compared with best supportive care (BSC),
chemotherapy is more beneficial for patients with AGC in terms of
being able to alleviate symptoms and prolong survival (18,19).
Based on these published studies, the median PFS was 4–6 months,
and the median OS was 7–13 months for patients who received
chemotherapy. In the present study, similar results were obtained:
The median PFS was 168 days in first-line chemotherapy, 96 days in
second-line chemotherapy, and the median OS was 356 days. The
further subgroup analyses yielded notable results. These disclosed
that OS was significantly longer for the following two groups:
Esophagogastric junction tumor site (Fig. 2A), and lymphatic metastasis (Fig. 2B). The result of the former subgroup
is not consistent with previous reports, indicating that
esophagogastric junction gastric cancer has poor prognosis. This
inconsistency could be explained by the small number of cases, and
the advances in nutritional treatment for patients with
esophagogastric lesions that are currently being made. The results
of the latter subgroup revealed that patients with lymphatic
metastasis had improved survival rates compared with patients with
peritoneal and hematogenous metastasis, and patients with a mixture
of metastases had comparatively the worst survival. The
histological factor appeared to be associated with OS, although the
difference was not significant, which may be attributed to an
insufficient number of cases in the present study. Other factors,
including gender, age, and whether a radical operation was
received, had no clear correlation with survival (Table I).
In addition, several of the treatment conditions,
including treatment with >2 lines of chemotherapy (Fig. 2C), increasing the number of cycles of
chemotherapy (Fig. 2D and E), and
good short-term efficacy of chemotherapy (Fig. 2F and G), led to markedly longer OS
times. These observations highlighted that, if patients have a good
tolerance to chemotherapy, they ought to be provided with more
opportunities for chemotherapy. Furthermore, the administration of
more drugs in combination may result in longer OS rates; although
no significant differences were identified in the present study,
this may have been due to the small number of cases involved.
However, regimens with larger numbers of drugs may result in more
severe side-effects. The National Comprehensive Cancer Network
(NCCN) guidelines from 2015 (https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#gastric)
recommend that two-drug cytotoxic regimens are preferred as the
first-line therapy, and the second-line therapy should be dependent
on prior therapy and performance status. In the present study,
two-drug cytotoxic regimens were routinely used (84.8% in the
first-line, and 72.2% in the second-line).
The most commonly used drugs in the treatment of AGC
include oxaliplatin, taxanes, and irinotecan. Alternatively,
fluoropyrimidine and cisplatin are often administered as basic
drugs. In the first-line chemotherapy, three groups, including
oxaliplatin-containing, taxane-containing and other regimens, had
no clear correlation with survival. A randomized phase III study
(AIO) demonstrated that irinotecan was able to prolong the OS in
patients with AGC whose first-line treatment failed, compared with
the BSC (13). Therefore,
irinotecan-contained regimens were compared with other regimens as
a second-line therapy. The median PFS was 90 days for the
irinotecan-containing regimen group, 73 days for the
oxaliplatin-containing regimen group, 105 days for the
taxane-containing regimen group, and 94 days for the ‘other
regimens’ group. No significant differences were identified among
the different groups.
The current study had certain limitations.
Conducting a retrospective analysis resulted in several differences
between the numbers of patients in each of the groups. The
toxicities were not recorded in detail. Nevertheless, the present
study remains of certain consultative value for the performing of
subsequent large-scale prospective clinical trials, and further
validation of the conclusions reported in the present study are
eagerly anticipated.
Acknowledgements
The presentß work was sponsored by the 1022 Talent
Training Program of Zhejiang Cancer Hospital, the General Research
Program of Medical Health in Zhejiang Province (grant nos.
2011KYA032, 2014KYB039 and 2016KYB036), the Scientific Research
Fund Project of the Integrated Chinese and Western Medicine
Institute in Zhejiang Province (no. 2014LYK021), the Science and
Technology in Zhejiang Province Chinese Medicine Program (nos.
2012ZA101, 2015ZA148 and 2016ZA038), the Hangzhou City Science and
Technology Project Planning Guide (Social Development; no.
20130733Q15), the Hangzhou City Health Science and Technology
Project (grant no. 2013A43) and the Public Welfare Technology
Application Studies Program of Zhejiang (grant no. 2015C33286).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai JY and Safran H: Status of treatment
for advanced gastric carcinoma. Curr Oncol Rep. 5:210–218. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
5
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: ACTS-GC Group: Adjuvant chemotherapy for
gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med.
357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, et
al: Fluorouracil versus combination of irinotecan plus cisplatin
versus S-1 in metastatic gastric cancer: A randomised phase 3
study. Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato Y, Takayama T, Sagawa T, Takahashi Y,
Ohnuma H, Okubo S, Shintani N, Tanaka S, Kida M, Sato Y, et al:
Phase II study of S-1, docetaxel and cisplatin combination
chemotherapy in patients with unresectable metastatic gastric
cancer. Cancer Chemother Pharmacol. 66:721–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi H, Kitayama J, Ishigami H, Emoto
S, Yamashita H and Watanabe T: A phase II trial of intravenous and
intraperitoneal paclitaxel combined with S-1 for treatment of
gastric cancer with macroscopic peritoneal metastasis. Cancer.
119:3354–3358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase III open-label randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oki E, Emi Y, Kusumoto T, Sakaguchi Y,
Yamamoto M, Sadanaga N, Shimokawa M, Yamanaka T, Saeki H, Morita M,
et al: Phase II study of docetaxel and S-1 (DS) as neoadjuvant
chemotherapy for clinical stage III resectable gastric cancer. Ann
Surg Oncol. 21:2340–2346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomized controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thuss-Patience PC, Kretzschmar A, Bichev
D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer-a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang JH, Lee SI, Lim do H, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW,
Baek SK, Kim TY, Ryu MH, Nam BH and Zang DY: Second-line
chemotherapy versus supportive cancer treatment in advanced gastric
cancer: A meta-analysis. Ann Oncol. 24:2850–2854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu T and Saijo N: Common toxicity
criteria: Version 2.0, an improved reference for grading the
adverse reaction of cancer treatment. Nihon Rinsho. 61:937–942.
2003.PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glimelius B, Hoffman K, Haglund U, Nyrén O
and Sjödén PO: Initial or delayed chemotherapy with best supportive
care in advanced gastric cancer. Ann Oncol. 5:189–190. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|