Introduction
Lung cancer was the leading cause of
cancer-associated death in the US in 2015. An estimated 221,000 new
cases of lung cancer were diagnosed, and 158,000 cancer-associated
mortalities were recorded (1).
Although smoking (90%) represents the highest risk factor for lung
cancer (2), only 10–15% of smokers
are diagnosed with lung cancer, which indicates that other
environmental factors, including asbestos, chromium, nickel,
beryllium, arsenic, silica, cadmium, radon, chronic obstructive
pulmonary disease, and genetic factors also serve a key role in the
etiology of the disease (3).
The American Joint Committee on Cancer (AJCC)
clinical staging system is the most widely accepted tool in the
evaluation of prognosis and survival of lung cancer (4). Serum albumin, C-reactive protein (CRP),
hemoglobin, lactate dehydrogenase (LDH), the leukocyte count,
interleukin (IL)-6, and tumor necrosis factor-α (TNF-α) levels have
been also shown to serve a predictive role (5). Furthermore, epidermal growth factor
receptor, anaplastic lymphoma kinase, proto-oncogene
tyrosine-protein kinase and Kirsten rat sarcoma 2 viral oncogene
homolog mutational analyses have gained in importance in recent
years, as they are helpful in terms of planning the treatment
(6).
In addition, IL-1 is a versatile cytokine, which has
unique physiological roles, including cytokine secretion in
autoimmune diseases, vascular permeability, and induction of fever
in sepsis (7). Previous studies have
revealed that increased IL-1 secretion enhances the expression of
genes that encode proteins involved in metastasis [i.e., matrix
metalloproteinases (MMPs)], and secretion of growth and angiogenic
factors, including vascular endothelial growth factor (VEGF), IL-8,
IL-6, TNF-α and transforming growth factor-β (8,9).
Furthermore, IL-1 receptor antagonist (IL-1Ra) is a member of the
IL-1 family, having homology with IL-1α and IL-1β (10). IL-1Ra binds to IL-1 receptors in a
competitive manner without exerting biological activity. It is
naturally produced, and competitively inhibits IL-1RI in
T-lymphocytes and fibroblasts (11).
Due to its ability to inhibit collagenase and prostaglandin
synthesis, the recombinant version of IL-1Ra, anakinra, has been
developed for the treatment of rheumatoid arthritis. Anakinra is
able to reverse the IL-1-dependent effects (11). IL-1 induces tumor growth and
metastasis (12), whereas, by
contrast, IL-1Ra inhibits IL-1α and IL-6 secretion in cancer cells.
The use of anakinra for the treatment of rheumatoid arthritis has
revealed that the drug is well absorbed, with a safe side-effect
profile, which makes it a good candidate for the treatment of
cancer types caused by inflammation. Therefore, a number of studies
have been performed to investigate whether IL-1Ra inhibits the
effects of IL-1 and is effective as a treatment of choice in cancer
therapy (8,12). These studies have demonstrated that
IL-1Ra suppresses metastasis and tumor proliferation, thereby
inhibiting synthesis of angiogenic factors, such as VEGF and IL-8
(12).
The present study aimed to investigate the possible
association between the serum IL-1Ra level, overall survival (OS),
and treatment response in advanced non-small cell lung cancer
(NSCLC), and to evaluate the usefulness of the serum IL-1Ra level
as a prognostic marker for NSCLC.
Patients and methods
A total of 80 chemotherapy-naive patients who were
admitted to the Department of Medical Oncology, Pamukkale
University, Denizli, Turkey for the first time and who were
diagnosed with NSCLC following a pathological examination were
included. Written informed consent was obtained from each patient.
The study protocol was approved by the Ethics Committee of
Pamukkale University, Faculty of Medicine, Clinical Research (dated
20.09.2010/05). The present study was conducted in accordance with
the principles of the Declaration of Helsinki. Only patients with
an advanced disease (stage IIIB or stage IV) with a Performance
Status (PS) of 0, 1, and 2, according to the World Health
Organization (WHO), were enrolled. Exclusion criteria were as
follows: a PS of ≤3 at the time of admission; brain metastasis or
suspected brain metastasis; age >80 years; and early-stage
disease (stage I, II, or IIIA). The control group consisted of 40
healthy individuals aged between 50 and 71 years, who did not use
any medication and who did not have any known diseases.
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 software (SPSS Inc., Chicago, IL, USA). A cut-off
value for IL-1Ra was calculated as 10 ng/ml. Values that were equal
to or lower than the cut-off value were considered low, whereas
values exceeding the cut-off value were considered high. The
chi-square and Mann-Whitney U tests were used to compare the
characteristics of the patients with NSCLC and healthy controls.
The Spearman and Pearson's correlation tests were used for the
correlation analysis. Kaplan-Meier survival plot analysis was used
to calculate OS, progression-free survival (PFS), and survival
curves. Cox regression analysis was used to determine the factors
affecting the survival of patients and progression of the disease.
A confidence interval of 95% was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic and clinical characteristics of the
patients and controls are shown in Table
I.
 | Table I.Demographic and clinical features of
patients with non-small cell lung cancer and control subjects. |
Table I.
Demographic and clinical features of
patients with non-small cell lung cancer and control subjects.
| Feature | Patients (n=80) | Controls (n=40) | P-value |
|---|
| Age (years) | 64.1±8.9 | 59.5±5.6 | 0.41 |
| Gender
(male/female) | 72/8 | 13/27 |
<0.001a |
| Hemoglobin
(g/dl) | 12.61±1.7 | 13.61±1.34 | 0.030a |
| WBC (K/µl) | 11,342±6,634 | 7,033±1,742 |
<0.001a |
| PNLs (K/µl) | 8,011±6,366 | 4,008±1,322 |
<0.001a |
| PLTs (K/µl) | 365,742±139,623 | 247,000±52,728 |
<0.001a |
| Albumin (g/dl) | 4.02±0.49 | 4.45±0.33 |
<0.001a |
| CRP (mg/dl) | 6.55±7.03 | 0.36±0.65 |
<0.001a |
| LDH (U/l) | 282±29.02 | 188±7.7 | 0.008a |
| Ferritin (ng/ml) | 398.76±78.7 | 63.4±10.03 |
<0.001a |
| IL-1Ra | 8.85±4.99 | 8.07±5.13 | 0.542 |
| Clinical features of
patients (%) |
|
|
|
|
Adenocarcinoma | 16 (20) |
|
|
|
Squamous-cell carcinoma | 54 (67.5) |
|
|
| Other
histological subtypes | 10 (12.5) |
|
|
|
Smokers | 72 (90) |
|
|
|
Experienced weight loss at
diagnosis | 44 (55) |
|
|
Survival was shorter in patients with high serum
IL-1Ra levels (38 weeks), compared with the patients with low serum
IL-1Ra levels (62 weeks; P=0.065). In addition, serum IL-1Ra levels
had an impact on progression. The time until progression was
significantly shorter in patients with high serum IL-1Ra levels (16
weeks) compared with the patients with low serum IL-1Ra levels (35
weeks; P=0.027).
Cox regression analysis revealed that albumin and
ferritin levels had a significant impact on progression (P=0.042
and P=0.001, respectively). The Cox regression analysis for PFS is
shown in Table II.
 | Table II.Cox regression analysis to determine
factors affecting progression-free survival. |
Table II.
Cox regression analysis to determine
factors affecting progression-free survival.
|
| Exp(B) | P-value | 95% CI |
|---|
| Albumin | 0.557 | 0.042a | 0.317–0.317 |
| Ferritin | 1.001 | 0.001a | 1.000–1.000 |
Multivariate analysis showed that the albumin level,
the elevated CRP level, and the level of IL-1Ra had an impact on OS
(P=0.019, 0.05 and 0.04, respectively). Low albumin levels were
associated with a 3-fold decrease in OS, whereas high CRP levels
were associated with a 2.9-fold decrease in OS. High serum levels
of IL-1Ra were associated with a 2.2-fold decrease in OS. Cox
regression analysis for OS is shown in Table III.
 | Table III.Cox regression analysis for overall
survival. |
Table III.
Cox regression analysis for overall
survival.
|
| Exp(B) | P-value | 95% CI |
|---|
| Albumin | 3.034 | 0.019a | 1.326–6.326 |
| CRP | 2.908 | 0.05a | 1.279–6.279 |
| IL-1Ra | 2.284 | 0.04a | 1.279–4.279 |
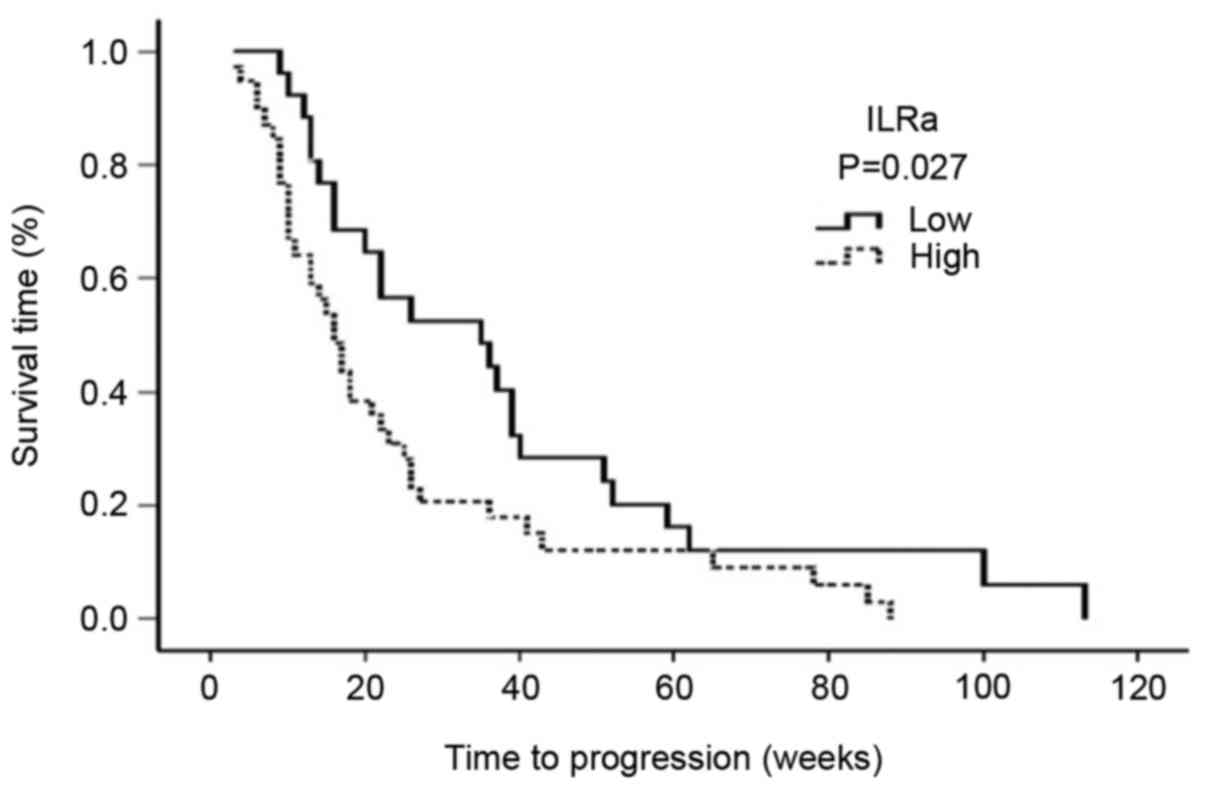
The serum levels of IL-1Ra exhibited an inverse
correlation with the median PFS. The median PFS was 16 weeks (95%
CI: 12–19 weeks) for patients with high serum IL-1Ra levels, and 35
weeks for patients with low serum IL-1Ra levels (95% CI: 12–57
weeks; P=0.027) (Fig. 1).
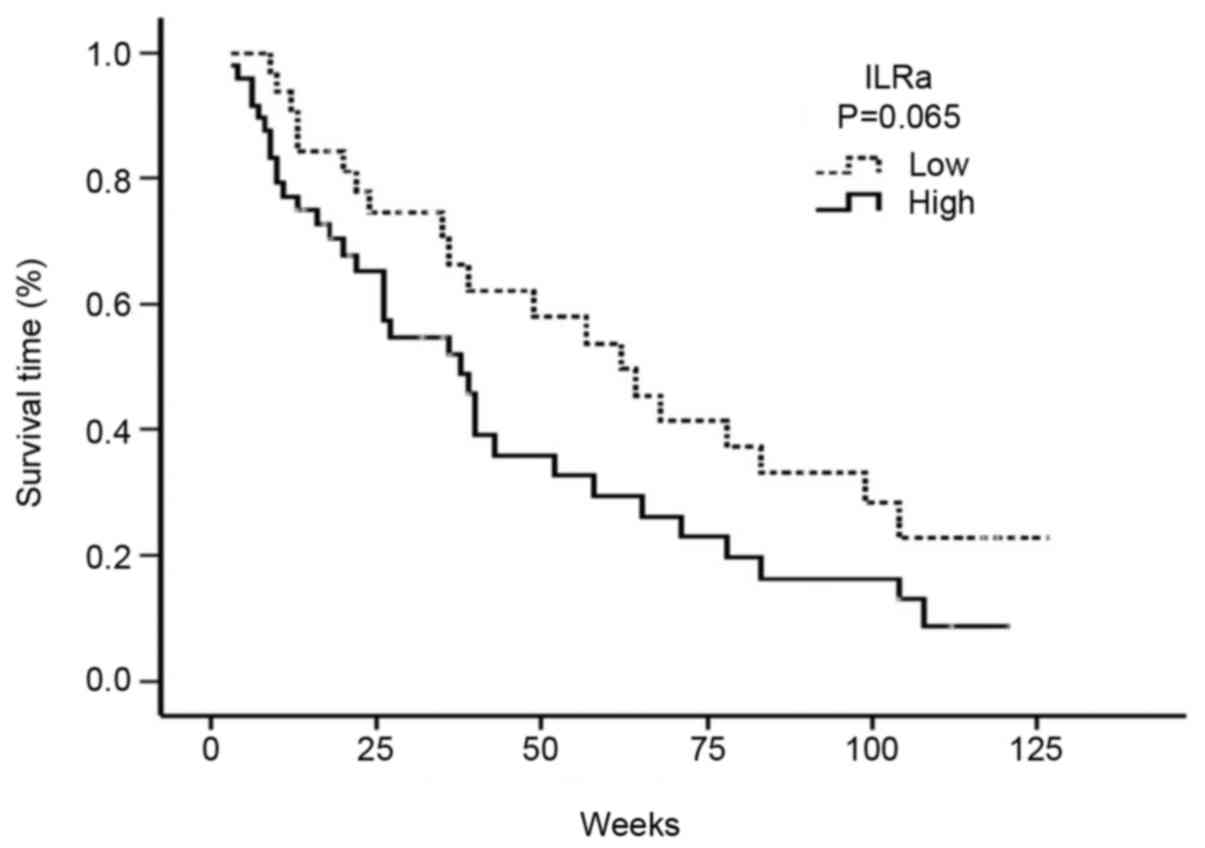
High serum levels of IL-1Ra were associated with a
decreased median OS. Accordingly, the median OS was 38 weeks (95%
CI: 26.05–49.94) in patients with a high serum IL-1Ra level, and 62
weeks (95% CI: 40.0–83.9) in patients with a low serum IL-1Ra level
(P=0.065) (Fig. 2).
Discussion
IL-1, which is a versatile cytokine, exerts a key
role not only in physiological processes, but also in pathological
processes, such as those concerned with autoimmune diseases,
sepsis, and malignancies (12). The
IL-1 family consists of pro-inflammatory and immunoregulatory
cytokines, including IL-1α, IL-1β and IL-1Ra (13). IL-1α and IL-1-β act as agonists,
whereas IL-1Ra has antagonistic effects. Although IL-1α and IL-1-β
are encoded on different genes, they bind to the identical receptor
with similar functions (11).
Despite sharing the same biological function, however, IL-1α is
localized in the cytoplasm or cell membrane and serves a key role
in intracellular regulation (11).
By contrast, IL-1β is first processed by IL-1β converting enzyme,
and the resulting mature form is subsequently secreted. The
increase in IL-1β concentration in case of an infection or
inflammation, rather than of IL-1α, suggests that IL-1β exerts
systemic effects (11).
IL-1Ra inhibits the inflammatory and
tumor-activating properties of IL-1 (12). In vivo studies on mice
revealed that IL-1Ra suppresses angiogenesis, tumor growth, and
metastasis (13). IL-1Ra levels are
also raised in autoimmune disorders in which the levels of IL-1 are
increased, such as metabolic diseases, sepsis, and cancer;
therefore, the IL-1Ra level may be used as an indicator of IL-1
activity (12).
Previous studies on IL-1 have demonstrated that IL-1
acts as a tumor growth factor, and increases proliferation of tumor
cells (11). In addition, IL-1 is
known to induce metastasis-inducing molecules (e.g., MMPs) and
angiogenic molecules (e.g., VEGF) (8). Angiogenesis serves a key role in
tumor-associated inflammation, tumor growth, and metastasis.
The protein expression of IL-1Ra in lung cancer
tissues is markedly higher compared with that of normal lung tissue
(14). This finding is interpreted
as an ‘escape mechanism’ from host defense systems.
In the present study, it has been shown that the
levels of serum IL-1Ra were higher in patients with NSCLC compared
with the control subjects; however, the difference between the
groups was not statistically significant. Similarly to our results,
a previous study revealed that IL-1Ra levels were higher in
patients with thyroid cancer (15).
In normal cells, IL-1 secretion is mediated via inflammatory
stimulation or cytokines, whereas tumor cells synthesize and
secrete IL-1 through an autocrine mechanism. In contrast with the
situation in normal cells, tumor cells exhibit non-selective IL-1
secretion. IL-1 is rarely secreted from normal cells; however, it
is continuously synthesized in different cancer cells (7). Inflammation is frequently investigated
in the etiology of different cancer types, including thyroid
cancer. The immune system is hyperactive in Hashimoto's
thyroiditis, and an association between this disease and thyroid
cancer has been reported (15).
In addition, a complex association exists between
IL-1Ra and the severity of inflammation. Studies on different
cancer types (including pancreatic cancer and breast cancer) have
revealed that high levels of IL-1Ra are correlated with the
severity of disease (16,17). On the other hand, a negative
correlation between IL-1Ra levels and disease severity was
identified in the case of acute myeloid leukemia (17). These findings may be interpreted as
follows: An increase in the serum levels of IL-1Ra is able to
reduce the tumor-aggravating effects of IL-1; in this case, it is
also able to decrease the severity of the disease. However, in
certain cancer types, IL-1RA may fail to reduce these effects, and
the tumor prognosis may consequently become worse, despite the
presence of high serum IL-1Ra levels (17).
Danis et al (18) demonstrated that IL-1 receptor
antagonist gene allele 2 (ILRN*2) polymorphism led to increased
levels of IL-1Ra and a decrease in IL-1α synthesis. High IL-1Ra
levels and inflammation are considered to decrease the removal of
carcinogenic polycyclic hydrocarbons, which are taken into the body
following exposure to smoking. Hu et al (19) suggested that the ILRN*2 polymorphism
was associated with a decreased risk of lung cancer. This
functional polymorphism affects serum IL-1Ra levels and exerts a
role in the immune response and in cancer risk. In a previous
study, the effect of polymorphism on cancer risk was compared
between patients with a lung cancer diagnosis and healthy control
subjects. The presence of this polymorphism was associated with a
32% decrease in the risk of lung cancer, and, in particular, a 47%
decrease was observed in the non-smoking group (19). A subsequent study revealed that the
IL-1Ra gene polymorphism alone did not increase cancer risk, but
that the cancer risk was increased when the IL-1β gene polymorphism
was also present (20). According to
a Norwegian study, carrying a homozygous ILRN*1 allele and the
IL-1B-31T allele together increases the risk of NSCLC by 3.08-fold
(21).
McKeown et al (22) compared serum levels of IL-1RA and
other inflammatory cytokines between patients with NSCLC and
healthy control subjects. These authors demonstrated that the serum
levels of IL6, IL8, CRP, and IL-1Ra were significantly higher in
the NSCLC group compared with the control group (22). In addition, these authors also
identified a significant correlation between serum IL-6 and IL-1Ra
levels, and serum CRP levels. These findings suggested that, in
addition to the serum level of CRP, the serum level of IL-1Ra may
also serve as a prognostic marker for NSCLC.
In a comparative study, 68 inflammation markers in
the circulation were analyzed among patients with lung cancer, who
were enrolled in a follow-up study along with patients with
prostate cancer, colorectal cancer, ovarian cancer, and healthy
control subjects (2). Among these
parameters, high levels of serum IL-1Ra were associated with a 29%
decreased risk of lung cancer. Subgroup analyses revealed no
significant differences existed between individuals who had never
smoked (P=0.25) and those who were active smokers (P=0.71), whereas
a significant difference was observed in ex-smokers (P<0.001).
In that study, serum samples were collected 2.9 years (median)
prior to the diagnosis of lung cancer. Therefore, monitoring the
serum IL-1Ra levels in individuals who are at risk of lung cancer
(e.g. smokers, and sufferers of pulmonary fibrosis, chronic
obstructive pulmonary disease, and chronic pulmonary infections)
may be useful for an early diagnosis of lung cancer.
Furthermore, previous studies on IL-1Ra have
revealed that tissue levels of IL-1Ra/IL-1β are negatively
correlated with VEGF in patients with colorectal cancer, and IL-1Ra
inhibits VEGF and reduces invasion (7). As VEGF enhances angiogenesis, and leads
to a poor tumor prognosis, anti-VEGF antibodies (e.g., bevacizumab)
are used for the treatment of lung cancer. Infusion treatment with
an IL-1Ra analog (anakinra) is used for patients with rheumatoid
arthritis, and this has a safe side-effect profile. The combination
of agents which inhibit the effects of IL-1 and reduce the VEGF
secretion (e.g., anakinra) with bevacizumab may also lead to an
increase in treatment efficacy, leading to reduced toxicity due to
lower doses (7).
In a further study, Herfs et al (23) exposed the rats to cigarette smoke for
16 h, and demonstrated that treatment with anakinra led to a marked
reduction in epithelial hyperplasia, and eliminated the development
of squamous metaplasia, which is a pre-malignant lesion. Thus, the
authors suggested that anti-IL-1Ra treatment for chronic
obstructive pulmonary disease may serve a useful role in prevention
of lung cancer through epithelial remodeling (23).
Previous studies on patients with estrogen
receptor-negative (ER-) breast cancer have demonstrated that IL-1Ra
negativity is a prognostic factor. Angiogenesis is a critical
process for breast cancer growth, and VEGF is able to induce
angiogenesis and accelerate tumor growth, thereby leading to a poor
prognosis. IL-1 enhances angiogenesis by inducing VEGF expression
(17). It appears that the
expression level of IL-1Ra increases when IL-1 levels are high;
however, this increase is not sufficient to inhibit the effects of
IL-1 in breast cancer.
In the study, PFS and OS were shorter in patients
with a level of high IL-1Ra, suggesting the presence of severe
inflammation in these patients. On the other hand, the high IL-1Ra
levels did not appear to serve any protective roles.
In conclusion, the present study has demonstrated
that there was a correlation between the levels of IL-1Ra and NSCLC
progression and survival, although the correlation between IL-1Ra
levels and the response to treatment was not statistically
significant. Given the safe side-effect profile of
IL-1Ra-associated drugs, and the importance of IL-1 in cancer, the
use of such drugs for cancer treatment appears to afford a feasible
approach. However, additional, well-designed, large-scale studies
are required to investigate the potential of IL-1Ra for cancer
treatment.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiels MS, Pfeiffer RM, Hildesheim A,
Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G,
Caporaso NE, et al: Circulating inflammation markers and
prospective risk for lung cancer. J Natl Cancer Inst.
105:1871–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Straif K, Benbrahim-Tallaa L, Baan R,
Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Guha N, Freeman C,
Galichet L, et al: WHO International Agency for Research on Cancer
Monograph Working Group: A review of human carcinogens--Part C:
Metals, arsenic, dusts, and fibres. Lancet Oncol. 10:453–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York: 2010
|
|
5
|
Yotsukura M, Ohtsuka T, Kaseda K, Kamiyama
I, Hayashi Y and Asamura H: Value of the Glasgow Prognostic Score
as a Prognostic Factor in Resectable Non-Small Cell Lung Cancer. J
Thorac Oncol. 11:1311–1318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sholl LM, Aisner DL, Varella-Garcia M,
Berry LD, Dias-Santagata D, Wistuba II, Chen H, Fujimoto J, Kugler
K, Franklin WA, et al: LCMC Investigators: Multi-institutional
Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung
Cancer Mutation Consortium Experience. J Thorac Oncol. 10:768–777.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dinarello CA: Biologic basis for
interleukin-1 in disease. Blood. 87:2095–2147. 1996.PubMed/NCBI
|
|
8
|
Konishi N, Miki C, Yoshida T, Tanaka K,
Toiyama Y and Kusunoki M: Interleukin-1 receptor antagonist
inhibits the expression of vascular endothelial growth factor in
colorectal carcinoma. Oncology. 68:138–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barillé S, Akhoundi C, Collette M,
Mellerin MP, Rapp MJ, Harousseau JL, Bataille R and Amiot M:
Metalloproteinases in multiple myeloma: Production of matrix
metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction
of MMP-1 by myeloma cells. Blood. 90:1649–1655. 1997.PubMed/NCBI
|
|
10
|
Apte RN and Voronov E: Interleukin-1--a
major pleiotropic cytokine in tumor-host interactions. Semin Cancer
Biol. 12:277–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dinarello CA: Interleukin-1 and
interleukin-1 antagonism. Blood. 77:1627–1652. 1991.PubMed/NCBI
|
|
12
|
Lewis AM, Varghese S, Xu H and Alexander
HR: Interleukin-1 and cancer progression: The emerging role of
interleukin-1 receptor antagonist as a novel therapeutic agent in
cancer treatment. J Transl Med. 4:482006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voronov E, Carmi Y and Apte RN: Role of
IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol.
601:265–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith DR, Kunkel SL, Standiford TJ,
Chensue SW, Rolfe MW, Orringer MB, Whyte RI, Burdick MD, Danforth
JM, Gilbert AR, et al: The production of interleukin-1 receptor
antagonist by human bronchogenic carcinoma. Am J Pathol.
143:794–803. 1993.PubMed/NCBI
|
|
15
|
Fuksiewicz M, Kaminska J, Kotowicz B,
Kowalska M, Rubach M and Pienkowski T: Serum cytokine levels and
the expression of estrogen and progesterone receptors in breast
cancer patients. Clin Chem Lab Med. 44:1092–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barber MD, Ross JA and Fearon KC: Changes
in nutritional, functional, and inflammatory markers in advanced
pancreatic cancer. Nutr Cancer. 35:106–110. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruserud O, Aasen I, Akselsen PE, Bergheim
J, Rasmussen G and Nesthus I: Interleukin 1 receptor antagonist
(IL1RA) in acute leukaemia: IL1RA is both secreted spontaneously by
myelogenous leukaemia blasts and is a part of the acute phase
reaction in patients with chemotherapy-induced leucopenia. Eur J
Haematol. 57:87–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danis VA, Millington M, Hyland VJ and
Grennan D: Cytokine production by normal human monocytes:
Inter-subject variation and relationship to an IL-1 receptor
antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol.
99:303–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Z, Shao M, Chen Y, Zhou J, Qian J, Xu
L, Ma H, Wang X, Xu Y, Lu D, et al: Allele 2 of the interleukin-1
receptor antagonist gene (IL1RN*2) is associated with a decreased
risk of primary lung cancer. Cancer Lett. 236:269–275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hurme M and Santtila S: IL-1 receptor
antagonist (IL-1Ra) plasma levels are co-ordinately regulated by
both IL-1Ra and IL-1β genes. Eur J Immunol. 28:2598–2602. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lind H, Zienolddiny S, Ryberg D, Skaug V,
Phillips DH and Haugen A: Interleukin 1 receptor antagonist gene
polymorphism and risk of lung cancer: A possible interaction with
polymorphisms in the interleukin 1 beta gene. Lung Cancer.
50:285–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McKeown DJ, Brown DJ, Kelly A, Wallace AM
and McMillan DC: The relationship between circulating
concentrations of C-reactive protein, inflammatory cytokines and
cytokine receptors in patients with non-small-cell lung cancer. Br
J Cancer. 91:1993–1995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herfs M, Hubert P, Poirrier AL, Vandevenne
P, Renoux V, Habraken Y, Cataldo D, Boniver J and Delvenne P:
Proinflammatory cytokines induce bronchial hyperplasia and squamous
metaplasia in smokers: Implications for chronic obstructive
pulmonary disease therapy. Am J Respir Cell Mol Biol. 47:67–79.
2012. View Article : Google Scholar : PubMed/NCBI
|