Introduction
Over 1.3 million individuals are annually diagnosed
with colorectal cancer (CRC) worldwide, and approximately half of
CRC patients eventually succumb to the disease (1). The metastatic lymph node status (N
classification) is currently considered the most reliable
prognostic indicator for patients with radically resected CRC
(2,3). In 1997 and 2002, the International
Union Against Cancer (UICC) and the American Joint Committee on
Cancer (AJCC) proposed a classification for N categories that was
based on the number of metastatic lymph nodes (4). Currently, this UICC/AJCC N
classification is used most widely for CRC staging. According to
the guidelines of the AJCC/UICC, a minimum of 12 lymph nodes must
be resected and assessed to adequately evaluate lymph node status.
The positive nodes category (pN), which is based on the number of
involved lymph nodes, may be affected by the adequacy of the lymph
nodes retrieved or examined (5) and
it is affected by age, site of disease, T stage, extensiveness of
lymphadenectomy performed by the surgeon and diligence of the
pathologist (6–8). Unfortunately, despite the AJCC
recommendation stating that ≥12 lymph nodes must be examined, the
median number of examined lymph nodes was low, ranging between 6
and 13 (9). If the number of
retrieved lymph nodes is <12, the pN category for those patients
may be inaccurate due to what is referred to as ‘stage migration’
or ‘Will Rogers phenomenon’ (10).
Recently, a new lymph node ratio (LNR)-based system
has been proposed, representing the ratio of the metastatic and
total retrieved lymph nodes. A number of studies have proven that
the LNR classification is superior to the UICC/AJCC number-based pN
classification, mainly because it is not as significantly affected
by the total number of retrieved nodes (11–16).
However, when the ratio of the metastatic lymph nodes is 0, it is
so regardless of the number of the total retrieved lymph nodes. It
is the same as the pN0 classification, in the sense that there are
no positive lymph nodes detected. A proportion of CRC patients have
no lymph node metastasis; those patients will not benefit from the
LNR classification in terms of predicting outcomes. The log odds of
positive lymph nodes (LODDS), another novel prognostic indicator,
was recently introduced (17) and it
is defined as the log of the ratio between the number of positive
and the number of negative lymph nodes. The prognostic significance
of LODDS in gastric, pancreatic and breast cancer was previously
investigated (18–22) and certain studies have indicated the
superiority of LODDS over LNR in terms of prognostic significance
in colon cancer (17,23,24). The
value of LODDS remains unclear, and studies comparing the
prognostic value among the pN, LNR and LODDS classifications for
CRC following R0 resection have been sparse. Thus, a study was
designed with the aim of evaluating LODDS as a prognostic factor
for CRC and comparing its prognostic value with those of the pN and
LNR classifications by analyzing a series of 192 patients submitted
to curative (R0) resection.
Patients and methods
Patients and follow-up
A total of 192 CRC patients who underwent curative
(R0) resection were recruited for the present study at Zhongnan
Hospital of Wuhan University (Wuhan, China) between January, 2007
and October, 2010. The exclusion criteria included stage IV
disease, administration of neoadjuvant chemotherapy, endoscopic
mucosal resection, synchronous or metachronous primary cancer in
other organs, patients for whom lymph node information was
unavailable and those for whom a complete follow-up was
unavailable. All the patients were followed up after surgery at 3-,
6- or 12-month intervals. The follow-up of the entire study
population was continued until either death or October, 2015. The
median follow-up period was 65 months (range, 4–106 months) for all
patients.
Definition of the three N
classifications
Lymph node involvement was classified according to
the seventh edition of the tumor-node-metastasis (TNM)
classification system of UICC/AJCC (N0, no metastasis; N1, 1–3
metastatic lymph nodes; and N2, >3 metastatic lymph nodes)
(25). LNR is defined as the ratio
of the metastatic and the total retrieved lymph nodes. LODDS was
estimated as follows: LODDS = log [(pnod + 0.5)/(tnod-pnod + 0.5)]
(23), where pnod is the number of
positive lymph nodes and tnod is the total number of retrieved
lymph nodes; 0.5 is added to both the numerator and the denominator
to avoid singularity. All the nodes were separately dissected from
the specimen at the end of the procedure by the surgeon.
Statistical computing and
analyses
To investigate the optimal categorization of LNR and
LODDS, the Classification and Regression Trees technique (CART)
(26) was used to determine high
discriminating cut-off points in the statistical R software
package, version 3.2.1 (The R Foundation for Statistical Computing,
Vienna, Austria). Statistical analyses were performed using SPSS
software, version 17.0 (SPSS Inc., Chicago, IL, USA). Several
clinicopathological characteristics were compared using the
Pearson's χ2 test or Fisher's exact test. Univariate
analysis of survival was performed using the Kaplan-Meier method to
estimate survival rates in patient subgroups and the log-rank test
was used to test differences among the survival curves of different
patient groups. A multivariate analysis was conducted by Cox
proportional hazards models. The 3-step multivariate analysis was
applied to identify the N classification most significantly
correlated with prognosis. In step 1 of the multivariate analysis,
all the significant factors in the univariate analysis were
included, as well as pN classification, excluding LNR and LODDS. In
step 2, LNR classification was also included, but not LODDS. In
step 3 of the multivariate analysis, all three N classifications
were included. To elucidate how LODDS is superior to the other two
N classifications, scatter plots were constructed. The cut-off
points were determined using the R statistical software package,
version 3.2.1., and statistical analyses were performed using SPSS
software, version 17.0. For all analyses, differences with P-values
<0.05 were considered statistically significant.
Results
Cut-off values and associations
between clinicopathological characteristics and LNR/LODDS
Of the 192 patients, 113 (58.9%) were male and 79
(41.1%) were female, and the median age was 59 years (range, 23–90
years). The median follow-up period was 65 months (range, 4–106
months) for all patients. The overall 5-year survival for the whole
group of patients was 71.5%. Among the 192 patients, the median
number of retrieved lymph nodes was 9 (range, 1–34) and the median
number of metastatic lymph nodes in node-positive patients was 1.1
(range, 1–18).
The optimal cut-off LNR and LODDS values were
calculated according to CART. As regards LNR, patients were grouped
as follows: LNR1, ratio <0.10; LNR2, ratio 0.10–0.33; and LNR3,
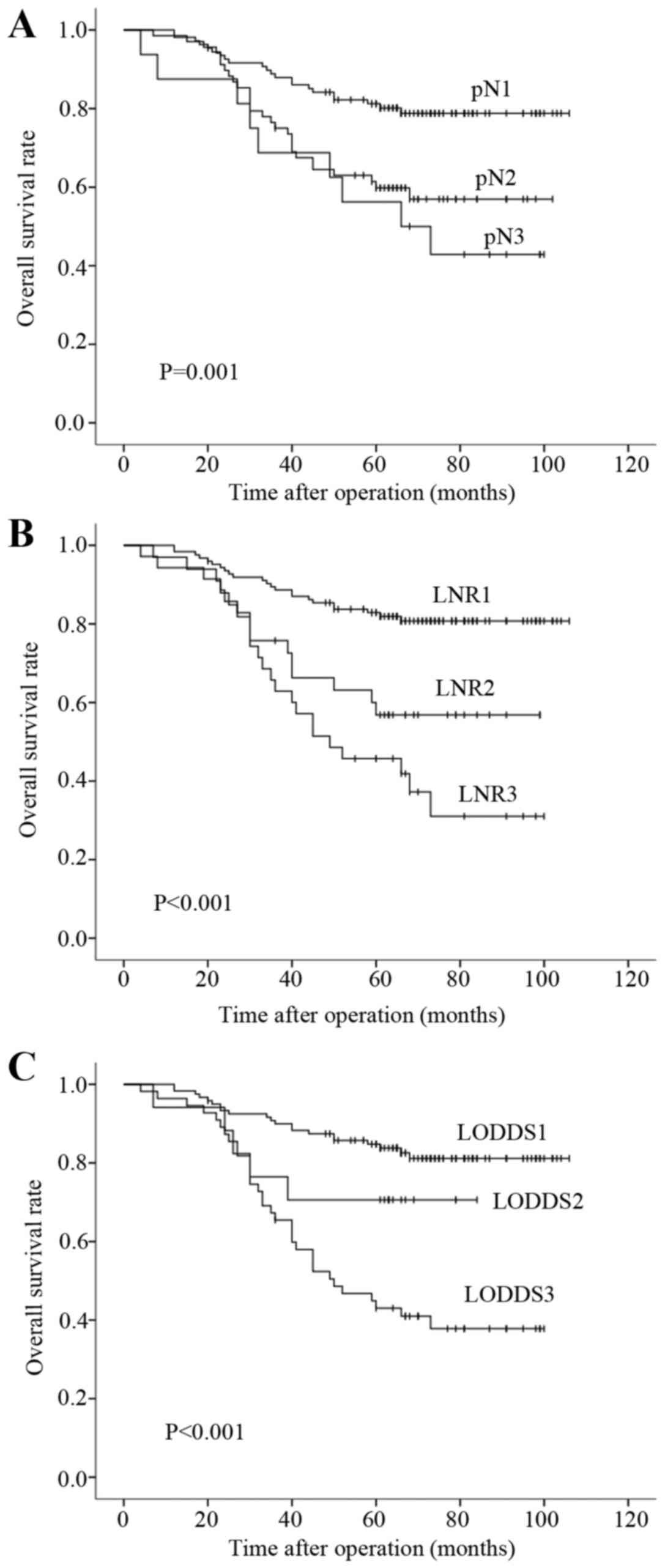
ratio ≥0.34. The 5-year survival rates in the LNR1, LNR2 and LNR3
groups were 82.9, 56.8 and 45.7%, respectively. Furthermore, the
patients were classified into three LODDS groups as follows: LODDS1
(LODDS<-0.82); LODDS2 (−0.82≤LODDS2<-0.57); and LODDS3
(LODDS≥-0.57). The 5-year survival rates of LODDS1, LODDS2 and
LODDS3 patients were 84.8, 70.6 and 43.0%, respectively. The 5-year
survival rates of pN0, pN1 and pN2 (pN classification) patients
were 81.2, 59.8 and 56.3% respectively.
The associations between clinicopathological
characteristics and the LNR/LODDS in this study are shown in
Table I. LNR classification was
connected with pN classification (P<0.001) and preoperative
serum carcinoembryonic antigen (CEA) level (P<0.05). The
proportion of higher pN classification increased with the
procession of LNR classification, as did the CEA level. This
association was also observed in the LODDS classification
(P<0.001 and P<0.05, respectively). In addition, LODDS was
significantly associated with age (P<0.05).
 | Table I.Associations between
clinicopathological characteristics and LNR/LODDS (n=192). |
Table I.
Associations between
clinicopathological characteristics and LNR/LODDS (n=192).
|
| LNR, n (%) | LODDS, n (%) |
|---|
|
|
|
|
|---|
| Characteristics | LNR1(n=124) | LNR2(n=33) | LNR3(n=35) | P-value | LODDS1(n=120) | LODDS2(n=17) | LODDS3(n=55) | P-value |
|---|
| Gender |
|
|
| 0.400 |
|
|
| 0.695 |
| Male | 75 (61) | 16 (49) | 22 (63) |
| 72 (60) | 11 (65) | 30 (55) |
|
Female | 49 (39) | 17 (51) | 13 (37) |
| 48 (40) | 6 (35) | 25 (45) |
| Age (years) |
|
|
| 0.429 |
|
|
| 0.041 |
|
<60 | 72 (58) | 18 (55) | 16 (46) |
| 71 (59) | 12 (71) | 23 (42) |
| ≥60 | 52 (42) | 15 (45) | 19 (54) |
| 49 (41) | 5 (29) | 32 (58) |
| Location |
|
|
| 0.071 |
|
|
| 0.399 |
| Left
colon | 12 (10) | 9 (27) | 5 (14) |
| 13 (11) | 2 (12) | 11 (20) |
| Right
colon | 33 (26) | 5 (15) | 5 (14) |
| 31 (26) | 3 (17) | 9 (16) |
|
Rectum | 79 (64) | 19 (58) | 25 (72) |
| 76 (63) | 12 (71) | 35 (64) |
| Max (cm) |
|
|
| 0.905 |
|
|
| 0.366 |
|
<5 | 94 (76) | 26 (79) | 26 (74) |
| 88 (73) | 15 (88) | 43 (78) |
| ≥5 | 30 (24) | 7 (21) | 9 (26) |
| 32 (27) | 2 (12) | 12 (22) |
|
Differentiation |
|
|
| 0.059 |
|
|
| 0.141 |
|
High | 35 (28) | 6 (18) | 5 (14) |
| 33 (27) | 4 (23) | 9 (16) |
|
Moderate | 75 (61) | 19 (58) | 20 (57) |
| 72 (60) | 11 (65) | 31 (57) |
|
Poor | 14 (11) | 8 (24) | 10 (29) |
| 15 (13) | 2 (12) | 15 (27) |
| T
stage |
|
|
| 0.075 |
|
|
| 0.152 |
| T1 | 3 (2) | 0 (0) | 0 (0) |
| 2 (2) | 0 (0) | 1 (2) |
| T2 | 40 (32) | 5 (15) | 7 (20) |
| 4 (33) | 2 (12) | 10 (18) |
| T3 | 44 (36) | 9 (27) | 16 (46) |
| 43 (36) | 6 (35) | 20 (36) |
| T4 | 37 (30) | 19 (58) | 12 (34) |
| 35 (29) | 9 (53) | 24 (44) |
| pN |
|
|
| <0.001 |
|
|
| <0.001 |
| N0 | 108 (87) | 0 (0) | 0 (0) |
| 100 (83) | 4 (24) | 4 (7) |
| N1 | 16 (13) | 29 (88) | 23 (66) |
| 20 (17) | 13 (76) | 35 (64) |
| N2 | 0 (0) | 4 (12) | 12 (34) |
| 0 (0) | 0 (0) | 16 (29) |
| Nodes
retrieved |
|
|
| 0.784 |
|
|
| 0.071 |
|
<12 | 79 (64) | 20 (61) | 24 (69) |
| 70 (58) | 14 (82) | 39 (71) |
|
≥12 | 45 (36) | 13 (39) | 11 (31) |
| 50 (42) | 3 (18) | 16 (29) |
| CEAa (ng/ml) |
|
|
| 0.038 |
|
|
| 0.024 |
|
<5 | 80 (74) | 18 (60) | 13 (50) |
| 77 (75) | 10 (67) | 24 (52) |
| ≥5 | 28 (26) | 12 (40) | 13 (50) |
| 26 (25) | 5 (33) | 22 (48) |
Survival analysis
In the univariate analysis (Table II), age, preoperative serum CEA
level, T stage, pN classification, LNR classification and LODDS
classification were found to be significantly correlated with
prognosis (P<0.05). The survival curves of patients according to
pN, LNR and LODDS classifications are presented in Fig. 1 (P=0.001, P<0.001 and P<0.001,
respectively), and exhibit a good discriminatory ability among
groups for all three N classifications.
 | Table II.Univariate survival analysis results
of 192 CRC patients. |
Table II.
Univariate survival analysis results
of 192 CRC patients.
| Variables | N(n=192) | 5-year OS rate
(%) | 95% CI | P-value |
|---|
| Gender |
|
|
| 0.696 |
|
Male | 113 | 70.4 | 70.3–70.3 |
|
Female | 79 | 73.1 | 73.0–73.0 |
| Age (years) |
|
|
| 0.002 |
|
<60 | 106 | 80.8 | 80.7–80.7 |
|
≥60 | 86 | 60.1 | 60.0–60.0 |
| Location |
|
|
| 0.438 |
| Left
colon | 26 | 61.0 | 60.8–61.8 |
| Right
colon | 43 | 71.0 | 70.9–71.9 |
|
Rectum | 123 | 73.9 | 73.8–74.8 |
| Max (cm) |
|
|
| 0.631 |
|
<5 | 146 | 70.2 | 70.1–70.1 |
| ≥5 | 46 | 75.8 | 75.7–75.7 |
|
Differentiation |
|
|
| 0.686 |
|
High | 46 | 78.3 | 78.2–78.2 |
|
Moderate | 114 | 69.6 | 69.5–69.5 |
|
Poor | 32 | 68.5 | 68.3–68.3 |
| T stage |
|
|
| 0.005 |
| T1 | 3 | 100.0 | 100.0–100.0 |
| T2 | 52 | 86.5 | 86.4–86.4 |
| T3 | 69 | 68.1 | 68.0–68.0 |
| T4 | 68 | 62.0 | 61.9–62.9 |
| Nodes
retrieved |
|
|
| 0.982 |
|
<12 | 123 | 71.3 | 70.5–72.5 |
|
≥12 | 69 | 71.9 | 71.8–72.8 |
| CEAa (ng/ml) |
|
|
| 0.001 |
|
<5 | 111 | 81.9 | 81.8–82.8 |
| ≥5 | 53 | 55.9 | 55.8–56.8 |
| pN |
|
|
| 0.001 |
| N0 | 108 | 81.2 | 81.1–81.1 |
| N1 | 68 | 59.8 | 59.7–59.7 |
| N2 | 16 | 56.3 | 56.1–56.1 |
| LNR |
|
|
| <0.001 |
|
LNR1 | 124 | 82.9 | 82.8–83.8 |
|
LNR2 | 33 | 56.8 | 56.6–57.6 |
|
LNR3 | 35 | 45.7 | 45.5–45.5 |
| LODDS |
|
|
| <0.001 |
|
LODDS1 | 120 | 84.8 | 84.7–84.7 |
|
LODDS2 | 17 | 70.6 | 70.4–70.4 |
|
LODDS3 | 55 | 43.0 | 42.9–43.9 |
3-step multivariate analysis
In step 1 of the multivariate analysis, pN
classification was confirmed to be an independent prognostic
factor. However, in step 2, LNR classification became significant,
while pN classification disappeared. Furthermore, when all the
three N classifications were included in step 3 of the multivariate
analysis, the pN and LNR classifications were substituted by the
LODDS classification (Table
III).
 | Table III.Multivariate analysis of factors by
Cox proportional hazards models. |
Table III.
Multivariate analysis of factors by
Cox proportional hazards models.
|
| Model 1 | Model 2 | Model 3 |
|---|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 1.866 | 1.044–3.044 | 0.035 | 1.669 | 0.935–2.935 | 0.083 | 1.587 | 0.885–2.885 | 0.121 |
| CEAa | 1.979 | 1.100–3.100 | 0.023 | 1.76 | 0.966–3.966 | 0.065 | 1.759 | 0.969–3.969 | 0.063 |
| T stage | 1.492 | 1.018–2.018 | 0.04 | 1.439 | 0.982–2.982 | 0.062 | 1.431 | 0.983–2.983 | 0.061 |
| pN | 1.589 | 1.053–2.053 | 0.027 | – | – | – | – | – | – |
| LNR | – | – | – | 1.746 | 1.252–2.252 | 0.001 | – | – | – |
| LODDS | – | – | – | – | – | – | 1.761 | 1.287–2.287 | <0.001 |
Scatter plots of the associations
among the three N classifications
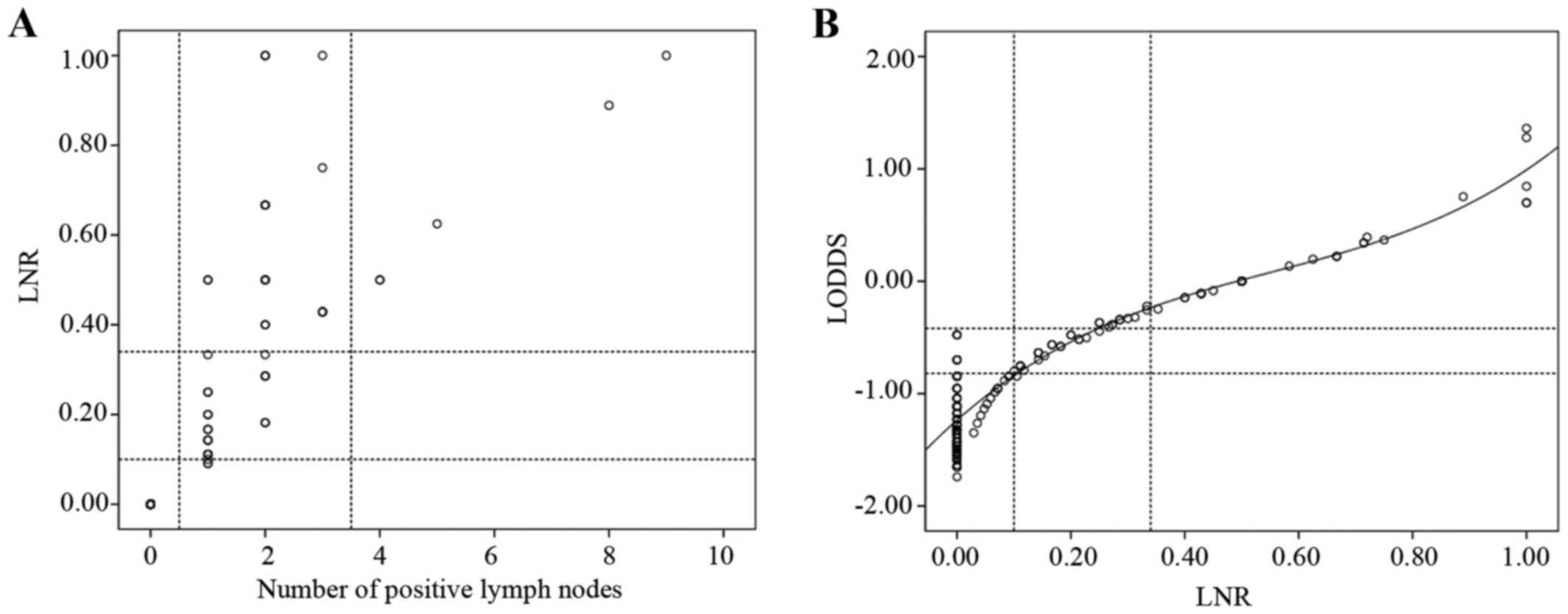
To demonstrate that the LODDS classification is
superior to the pN classification, scatter plots were constructed
(Fig. 2). When observing the
distribution of the number of metastatic nodes and the LNR of
patients whose number of retrieved lymph nodes was <12, LNR was
able to discriminate among patients with different prognoses in the
pN1 stage (Fig. 2A). The correlation
between the ratio of metastatic nodes and LODDS was significant
(Fig. 2B). When the LNR was <0.4,
it increased more slowly than LODDS. The most noteworthy result was
that, when the LNR was 0, the value of LODDS was still
heterogeneous.
Discussion
A series of 192 CRC patients submitted to curative
(R0) resection were analyzed in this study. To the best of our
knowledge, this study was the first to demonstrate that the LODDS
classification is the best prognostic factor in Chinese CRC
patients undergoing curative (R0) resection compared with the pN
and LNR N classifications.
The primary limitation of the number-based UICC/AJCC
pN classification is that the accuracy of predicting prognosis is
significantly affected by the total number of retrieved nodes
(15). Previous studies have
investigated the prognostic significance of LNR in stage III colon
cancer (5,27–29) and
stage III rectal cancer (30) and
suggested the LNR is useful because it provides information
regarding the number of retrieved lymph nodes. Although the LNR
classification has been proven to be superior to the pN
classification, there are limitations when the ratio of the
metastatic to the total retrieved lymph nodes is 0. LODDS, a novel
indicator of predicting lymph node status, may provide a new means
for improving the accuracy of N classification for prognostic
assessment.
Previous studies have indicated the superiority of
LODDS over LNR in terms of prognostic significance in colon cancer
(17,23,24).
However, the available data remain limited. The aim of this study
was to compare the prognostic value of pN, LNR and LODDS in CRC
patients who underwent R0 resection.
The LNR classification was correlated with the pN
classification (P<0.001) and preoperative serum CEA level
(P<0.05). The proportion of patients with a higher pN
classification increased with the procession of the LNR
classification. When the LNR classification increased, the
proportion of patients with preoperative serum CEA level ≥5 ng/ml
increased gradually. To some extent, this was in accordance with
the study of Nozoe et al (31), which demonstrated that the proportion
of lymph node metastasis was significantly higher in the high-CEA
group compared with that in low-CEA group (P=0.012). This
association was also observed in the LODDS classification.
Additionally, patients in the low age group were mainly in the
lower LODDS classification, while the gap was narrowed in the
higher age group; this result may be attributed to the poor
immunity of older patients.
Our univariate analysis demonstrated that each node
classification system had a relevant prognostic significance. To
investigate whether one N classification was superior to the
others, a multistep multivariate analysis was used. For example, to
compare the LODDS classification with the pN and LNR
classifications, a 3-step multivariate analysis was performed. In
step 1 of the multivariate analysis, pN classification was one of
the independent prognostic factors, whereas in step 2 the pN
classification was substituted by the LNR classification. In
addition, a 3-step multivariate analysis was performed, including
all three N classifications (pN, LNR and LODDS) and the LODDS
retained its significance (model 3). The results indicated that the
LODDS classification was superior to the pN and LNR
classifications.
To validate the superiority of the LODDS
classification and address its contribution to the accuracy of
prognostic assessment, several scatter plots were constructed. The
scatter plot presenting the distribution of the number of
metastatic nodes and LNR of patients with a number of retrieved
lymph nodes of <12, LNR was able to discriminate among patients
with different prognosis in the pN1 stage (Fig. 2A). For example, when the number of
positive lymph nodes was 1, it was classified as pN1 stage using
the pN classification, while it was divided into LODDS1, LODDS2 and
LODDS3 using the LODDS classification. LNR and LODDS were closely
correlated (Fig. 2B). When the ratio
of node metastasis was <0.4, it increased more slowly compared
with LODDS. It is intriguing that, when the ratio of node
metastasis was 0 or 1, the value of LODDS was still heterogeneous.
LODDS is more efficient in discriminating patients with different
survival, indicating that the LODDS system has the potential to
discriminate among patients with the same LNR classification with
different prognosis, particularly those whose ratio of node
metastasis is 0 or 1.
Wang et al (24) investigated 24,477 patients with stage
III colon cancer who were registered in the SEER database to
compare the LNR and LODDS classifications, and revealed that LODDS
was a better prognostic factor than LNR. The LODDS system was a
highly reliable staging system with strong predictive ability for
non-metastatic colon cancer patients (17,23). In
the present study, the LODDS classification was found to be
superior to the pN and LNR classifications in Chinese patients with
CRC undergoing R0 curative resection for the first time. Song et
al (32) revealed that, for
Chinese patients with CRC, the LNR classification was more suitable
compared with the pN and LODDS classifications for prognostic
assessment. Several reasons may have contributed to these different
results: i) The cut-off points acquired from different statistical
methods for subclassification were different; ii) the proportion of
colon and rectal cancer patients was different; and iii) the time
interval between the date of the last patient undergoing curative
resection to the follow-up deadline (October, 2015) was
different.
This study has some limitations, as it was
retrospective in nature and included a relatively limited number of
patients from one hospital in China. Larger-sample studies and
international multicentric research in CRC are required in the near
future.
In contrast to the UICC/AJCC pN and LNR
classifications, the LODDS system accurately estimates prognosis,
minimizing the bias of limited lymph node dissection and
examination. In conclusion, the results of the present study
suggest that LODDS may be a meaningful prognostic factor, which is
superior to the pN and LNR classifications in CRC patients who have
undergone R0 resection. Therefore, incorporating the LODDS
classification into the CRC staging system may enable clinicians to
assess the prognosis of patients more accurately.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (No. 81071825), the Doctoral
Fund of Ministry of Education of China (No. 20120141130010).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang GJ, Rodriguez-Bigas MA, Skibber JM
and Moyer VA: Lymph node evaluation and survival after curative
resection of colon cancer: Systematic review. J Natl Cancer Inst.
99:433–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ovrebo K and Rokke O: Extended lymph node
dissection in colorectal cancer surgery. Reliability and
reproducibility in assessments of operative reports. Int J
Colorectal Dis. 25:213–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greene FL: Current TNM staging of
colorectal cancer. Lancet Oncol. 8:572–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park IJ, Choi GS and Jun SH: Nodal stage
of stage III colon cancer: The impact of metastatic lymph node
ratio. Ann Surg Oncol. 100:240–243. 2009. View Article : Google Scholar
|
|
6
|
Ahmadi O, Stringer MD, Black MA and McCall
JL: Clinico-pathological factors influencing lymph node yield in
colorectal cancer and impact on survival: Analysis of New Zealand
cancer registry data. J Surg Oncol. 111:451–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonsalves WI, Kanuri S, Tashi T, Aldoss I,
Sama A, Al-Howaidi I, Ganta A, Kalaiah M, Thota R, Krishnamurthy J,
et al: Clinicopathologic factors associated with lymph node
retrieval in resectable colon cancer: A Veterans' Affairs Central
Cancer Registry (VACCR) database analysis. J Surg Oncol.
104:667–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong KP, Poon JT, Fan JK and Law WL:
Prognostic value of lymph node ratio in stage III colorectal
cancer. Colorectal Dis. 13:1116–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong SL, Ji H, Hollenbeck BK, Morris AM,
Baser O and Birkmeyer JD: Hospital lymph node examination rates and
survival after resection for colon cancer. JAMA. 298:2149–2154.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feinstein AR, Sosin DM and Wells CK: The
will rogers phenomenon. Stage migration and new diagnostic
techniques as a source of misleading statistics for survival in
cancer. N Engl J Med. 312:1604–1608. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceelen W, Van Nieuwenhove Y and Pattyn P:
Prognostic value of the lymph node ratio in stage III colorectal
cancer: A systematic review. Ann Surg Oncol. 17:2847–2855. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huh JW, Kim YJ and Kim HR: Ratio of
metastatic to resected lymph nodes as a prognostic factor in
node-positive colorectal cancer. Ann Surg Oncol. 17:2640–2646.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu YJ, Lin PC, Lin CC, Wang HS, Yang SH,
Jiang JK, Lan YT, Lin TC, Liang WY, Chen WS, et al: The impact of
the lymph node ratio is greater than traditional lymph node status
in stage III colorectal cancer patients. World J Surg.
37:1927–1933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moug SJ, Saldanha JD, McGregor JR,
Balsitis M and Diament RH: Positive lymph node retrieval ratio
optimises patient staging in colorectal cancer. Br J Cancer.
100:1530–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu HB, Zhang LY, Li YF, Zhou ZW, Keshari
RP and Xu RH: Ratio of metastatic to resected lymph nodes enhances
to predict survival in patients with stage III colorectal cancer.
Ann Surg Oncol. 18:1568–1574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenberg R, Engel J, Bruns C, Heitland W,
Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, et
al: The prognostic value of lymph node ratio in a population-based
collective of colorectal cancer patients. Ann Surg. 251:1070–1078.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arslan NC, Sokmen S, Canda AE, Terzi C and
Sarioglu S: The prognostic impact of the log odds of positive lymph
nodes in colon cancer. Colorectal Dis. 16:O386–O392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aurello P, Petrucciani N, Nigri GR, La
Torre M, Magistri P, Tierno S, D'Angelo F and Ramacciato G: Log
odds of positive lymph nodes (LODDS): What are their role in the
prognostic assessment of gastric adenocarcinoma? J Gastrointest
Surg. 18:1254–1260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
La Torre M, Nigri G, Petrucciani N,
Cavallini M, Aurello P, Cosenza G, Balducci G, Ziparo V and
Ramacciato G: Prognostic assessment of different lymph node staging
methods for pancreatic cancer with R0 resection: PN staging, lymph
node ratio, log odds of positive lymph nodes. Pancreatology.
14:289–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS,
Zhang DS, Luo HY, Li YH and Xu RH: The tumor-log odds of positive
lymph nodes-metastasis staging system, a promising new staging
system for gastric cancer after D2 resection in China. PLoS One.
7:e317362012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Z, Xu Y, de Li M, Wang ZN, Zhu GL,
Huang BJ, Li K and Xu HM: Log odds of positive lymph nodes: A novel
prognostic indicator superior to the number-based and the
ratio-based N category for gastric cancer patients with R0
resection. Cancer. 116:2571–2580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vinh-Hung V, Verschraegen C, Promish DI,
Cserni G, Van de Steene J, Tai P, Vlastos G, Voordeckers M, Storme
G and Royce M: Ratios of involved nodes in early breast cancer.
Breast Cancer Res. 6:R680–R688. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Persiani R, Cananzi FC, Biondi A, Paliani
G, Tufo A, Ferrara F, Vigorita V and D'Ugo D: Log odds of positive
lymph nodes in colon cancer: A meaningful ratio-based lymph node
classification system. World J Surg. 36:667–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Hassett JM, Dayton MT and Kulaylat
MN: The prognostic superiority of log odds of positive lymph nodes
in stage III colon cancer. J Gastrointest Surg. 12:1790–1796. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge S, Byrd DR, Compton C, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York: 2010
|
|
26
|
Breiman L, Friedman J, Stone CJ and Olshen
RA: Classification and Regression Trees. Chapman and Hall/CRC; New
York: 1984
|
|
27
|
Chen SL, Steele SR, Eberhardt J, Zhu K,
Bilchik A and Stojadinovic A: Lymph node ratio as a quality and
prognostic indicator in stage III colon cancer. Ann Surg.
253:82–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong KD, Lee SI and Moon HY: Lymph node
ratio as determined by the 7th edition of the American Joint
Committee on Cancer staging system predicts survival in stage III
colon cancer. J Surg Oncol. 103:406–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HY, Choi HJ, Park KJ, Shin JS, Kwon
HC, Roh MS and Kim C: Prognostic significance of metastatic lymph
node ratio in node-positive colon carcinoma. Ann Surg Oncol.
14:1712–1717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi H, Mochizuki H, Kato T, Mori T,
Kameoka S, Shirouzu K, Saito Y, Watanabe M, Morita T, Hida J, et
al: Lymph node ratio is a powerful prognostic index in patients
with stage III distal rectal cancer: A Japanese multicenter study.
Int J Colorectal Dis. 26:891–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nozoe T, Rikimaru T, Mori E, Okuyama T and
Takahashi I: Increase in both CEA and CA19-9 in sera is an
independent prognostic indicator in colorectal carcinoma. J Surg
Oncol. 94:132–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song YX, Gao P, Wang ZN, Tong LL, Xu YY,
Sun Z, Xing CZ and Xu HM: Which is the most suitable classification
for colorectal cancer, log odds, the number or the ratio of
positive lymph nodes? PLoS One. 6:e289372011. View Article : Google Scholar : PubMed/NCBI
|