Introduction
Biliary tract cancers (BTCs) include carcinomas of
the gallbladder, bile ducts (cholangiocarcinoma) and the papilla of
Vater. The majority of BTCs are detected at an advanced incurable
stage and are typically treated with chemotherapy, such as
5-fluorouracil (5-FU), gemcitabine (GEM), cisplatin, or their
combination. The response rates range between 20 and 40%, with a
median overall survival (OS) of 8–14 months (1). The most notable advance in the
treatment of BTC was achieved by a phase III randomized trial
demonstrating that doublet chemotherapy with GEM plus cisplatin
improved the OS by 3.6 months vs. GEM alone (2). However, the median OS with GEM plus
cisplatin is 11.7 months, and appropriate third-line chemotherapy
following GEM (plus cisplatin) and 5-FU is currently unavailable
for the treatment of patients with BTC.
Paclitaxel (PTX) is an anticancer agent (3) that stabilizes polymerized microtubules
and enhances microtubule assembly, arresting the cell cycle in
G0/G1 and G2/M and leading to cell death (4,5).
Low-dose PTX ameliorates tissue fibrosis by inhibiting the activity
of the transforming growth factor (TGF)-β/Smad activity (6,7).
Therefore, it was hypothesized that PTX may be useful for the
treatment of patients with BTC associated with tissue fibrosis.
It has been demonstrated that low-dose PTX inhibits
the epithelial-to-mesenchymal transition (EMT) of
cholangiocarcinoma cells treated with TGF-β (8). Furthermore, we previously reported the
response of a patient with gallbladder cancer with multiple liver
metastases and stenosis of the bile duct to treatment with low-dose
PTX as palliative chemotherapy following GEM and S-1 (oral prodrug
of 5-FU) (9). Therefore, a phase I
clinical trial was planned to determine the optimal dose of weekly
low-dose PTX therapy as third-line chemotherapy for patients with
BTC following failure of S-1 and GEM (plus cisplatin).
Patients and methods
Patient selection
Patients with unresectable (locally advanced or with
distant metastases) or postoperative recurrent BTC treated with GEM
(plus cisplatin) and S-1 were considered eligible for the study.
Other inclusion criteria were as follows: Age 20–80 years, Eastern
Cooperative Oncology Group performance status ≤1 (ambulatory and
capable of self-care), adequate renal function (normal serum
creatinine and blood urea nitrogen levels), liver function [total
bilirubin level, <2.5-times the upper limit of normal (ULN) or
<3-times the ULN following biliary drainage, if the patient had
jaundice and serum alanine aminotransferase and aspartate
aminotransferase levels <2.5-times the ULN or <3-times the
ULN following biliary drainage, if the patient had jaundice], bone
marrow reserve (white blood cell count, 4,000–12,000
mm3; neutrophil count, >2,000 mm3;
platelet count, >100,000 mm3; and hemoglobin level
>9.5 g/dl) and pulmonary function (PaO2, >70
mmHg). If a patient had a history of treatment for BTC, such
treatment (tumor resection, chemotherapy, immunotherapy or
radiotherapy) must have been discontinued ≥2 weeks prior to
enrolment.
The exclusion criteria were as follows: Pulmonary
fibrosis or interstitial pneumonia, marked pleural or pericardial
effusion or marked peripheral edema, severe heart disease,
difficult-to-control diabetes mellitus, active infection, pregnancy
or lactation, women of childbearing age who did not use effective
contraception, severe drug hypersensitivity, severe neurological
impairment or mental disorder, active concomitant malignancy,
previous history of PTX administration and other serious medical
conditions.
Written informed consent was obtained from each
patient prior to enrolment, and the study protocol was approved by
the Institutional Review Board of Kanazawa University Hospital
(UMIN ID: 000008148).
Study design
An open-label, single-center, non-randomized,
dose-escalation phase I study was conducted. The laboratory tests
to assess eligibility were completed within 7 days prior to
commencing treatment. PTX was administered as a 60-min intravenous
infusion on days 1, 8, 15 and 22 of each cycle. The cycle was
repeated twice every 28 days. The dose of PTX was planned as
follows: Level 1, 40 mg/m2; level 2,50 mg/m2
(Fig. 1). If the treatment was
deemed as effective after two cycles, PTX therapy was continued
weekly or biweekly for as long as possible.
Definition of dose-limiting toxicity
(DLT) and maximum tolerated dose (MTD)
DLT was determined during each treatment cycle and
was defined according to the National Cancer Institute's Common
Toxicity Criteria scale, version 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40)
as one or more of the effects attributable to the study drug as
follows: i) Grade 3/4 neutropenia complicated by fever; ii) grade 4
neutropenia for >4 days; iii) grade 4 thrombocytopenia; iv) any
other grade 3/4 non-hematological toxicity, apart from anorexia,
nausea and vomiting in the absence of an appropriate antiemetic;
and v) delay of recovery from treatment-related toxicity for >2
weeks. At least 3 patients were enrolled at each dose level. If DLT
was observed after the first cycle in >2 patients, treatment at
that dose was discontinued. If DLT was observed after the first
cycle in 1 patient, 3 additional patients were placed on that dose
level. If only 1 of 6 patients experienced DLT, dose escalation was
continued. The MTD of the combination was defined as the dose that
produced DLT in >2 of the 6 patients or in the 3 initial
patients. The recommended dose (RD) was defined as the dose one
level below the MTD, considering toxicity and tolerability to
outpatients. If level 2 was lower than the MTD, further dose
escalation was not performed, and level 2 became the RD, as the
intent was palliation.
Assessment of efficacy
Tumor response was evaluated according to the
Response Evaluation Criteria in Solid Tumors (10). Complete response (CR) was defined as
the disappearance of clinical evidence of the tumor. Partial
response (PR) was defined as a ≥30% reduction in the sum of the
products of two orthogonal diameters of all measurable lesions
compared with the baseline values, with no evidence of new lesions.
Stable disease (SD) was defined as <30% reduction or <20%
increase in the sum of the products of two orthogonal diameters of
all measurable lesions compared with baseline values, with no
evidence of new lesions. Progressive disease (PD) was defined as
≥20% increase in the sum of the products of two orthogonal
diameters of all measurable lesions compared with baseline values,
the appearance of a new lesion, or deterioration of clinical status
consistent with disease progression. To assess objective response,
the patients were evaluated after two cycles of chemotherapy, and
the concentrations of the tumor markers carcinoembryonic antigen
(CEA) and carbohydrate antigen (CA) 19–9 were measured before and
after two treatment cycles.
Statistical analysis
The median survival time (MST) and OS were
calculated from the treatment initiation until death from any cause
and were determined using the Kaplan-Meier method. The significance
of the differences in the data for CEA and CA19-9 concentrations
were evaluated using the paired t-test after logarithmic
transformation of the values and P<0.05 was considered to
indicate statistically significant differences. The SPSS
statistical package (version 19; SPSS Inc., Chicago, IL, USA) was
used to conduct the analyses.
Results
Patient characteristics
Between October, 2012 and May, 2013, a total of 6
patients (2 men and 4 women) diagnosed with BTC were enrolled in
the present study. The patient characteristics and the effects of
treatment are listed in Table I.
Cases 1–5 were patients with postoperative recurrent BTC and case 6
was a patient with locally advanced gallbladder cancer case with
liver metastases. Treatment was performed at level 1 (40
mg/m2) and level 2 (50 mg/m2) for 3 patients.
The response to treatment were as follows: PR, 3/6 (50.0%); SD, 2/6
(33.3%); and PD, 1/6 (16.7%). The disease control rate (PR + SD)
was 83.3%. During the two courses of therapy, grade 1 or 2 adverse
events were observed in all the patients; however, dose-limiting
adverse events (grade 3 or 4) were not observed, and all the
patients completed two courses of treatment. The adverse events
were as follows: Hair loss (100.0%), anemia (83.3%), general
malaise (66.7%), as well as thrombocytopenia, diarrhea and joint
pain (16.7%). Significant neuropathy and neutropenia were not
observed during the two treatment cycles (Table II).
 | Table I.Patient characteristics and treatment
effects. |
Table I.
Patient characteristics and treatment
effects.
| No. | Age, yrs | Gender | Primary tumor | Dose
(mg/m2) | Treatment effect | TTF (months) |
|---|
| 1 | 73 | Female | Gallbladder | 40 | PR | 4 |
| 2 | 65 | Female | Intrahepatic bile
duct | 40 | SD | 9 |
| 3 | 79 | Male | Cystic duct | 40 | PR | 14 |
| 4 | 80 | Female | Intrahepatic bile
duct | 50 | PD | 0 |
| 5 | 65 | Male | Hilar bile duct | 50 | SD | 5 |
| 6 | 57 | Female | Gallbladder | 50 | PR | 9 |
 | Table II.Adverse events. |
Table II.
Adverse events.
| Toxicity | Grade 1/2, n (%) | Grade 3/4 |
|---|
| Neutropenia | 0 (0.0) | 0 |
| Anemia | 4
(66.7) | 0 |
| Thrombocytopenia | 1
(16.7) | 0 |
| Anorexia | 0 (0.0) | 0 |
| General
malaise | 4
(66.7) | 0 |
| Diarrhea | 1
(16.7) | 0 |
| Neuropathy | 0 (0.0) | 0 |
| Joint pain | 1
(16.7) | 0 |
| Hair loss |
6 (100.0) | – |
The CEA concentrations prior to treatment were
elevated to >5 IU/ml (normal, ≤5 IU/ml) in 4 of the 6 patients,
and the CA-19-9 concentrations prior to treatment were elevated to
>37 IU/ml (normal, ≤37 IU/ml) in 4 of the 6 patients. The CEA
and CA19-9 concentrations decreased in 4 and 2 patients,
respectively, and the decrease in CEA concentration was significant
(P=0.039) (Table III).
 | Table III.Transition of tumor markers. |
Table III.
Transition of tumor markers.
| Tumor markers | Prior to treatment
(mean ± SE) | After two treatment
cycles (mean ± SE) | P-value |
|---|
| CEA (IU/ml) | 27.8 (±12.6) | 5.2
(±1.5) | 0.039 |
| CA19-9 (IU/ml) | 349.2 (±322.9) | 104.0 (±75.3) | 0.908 |
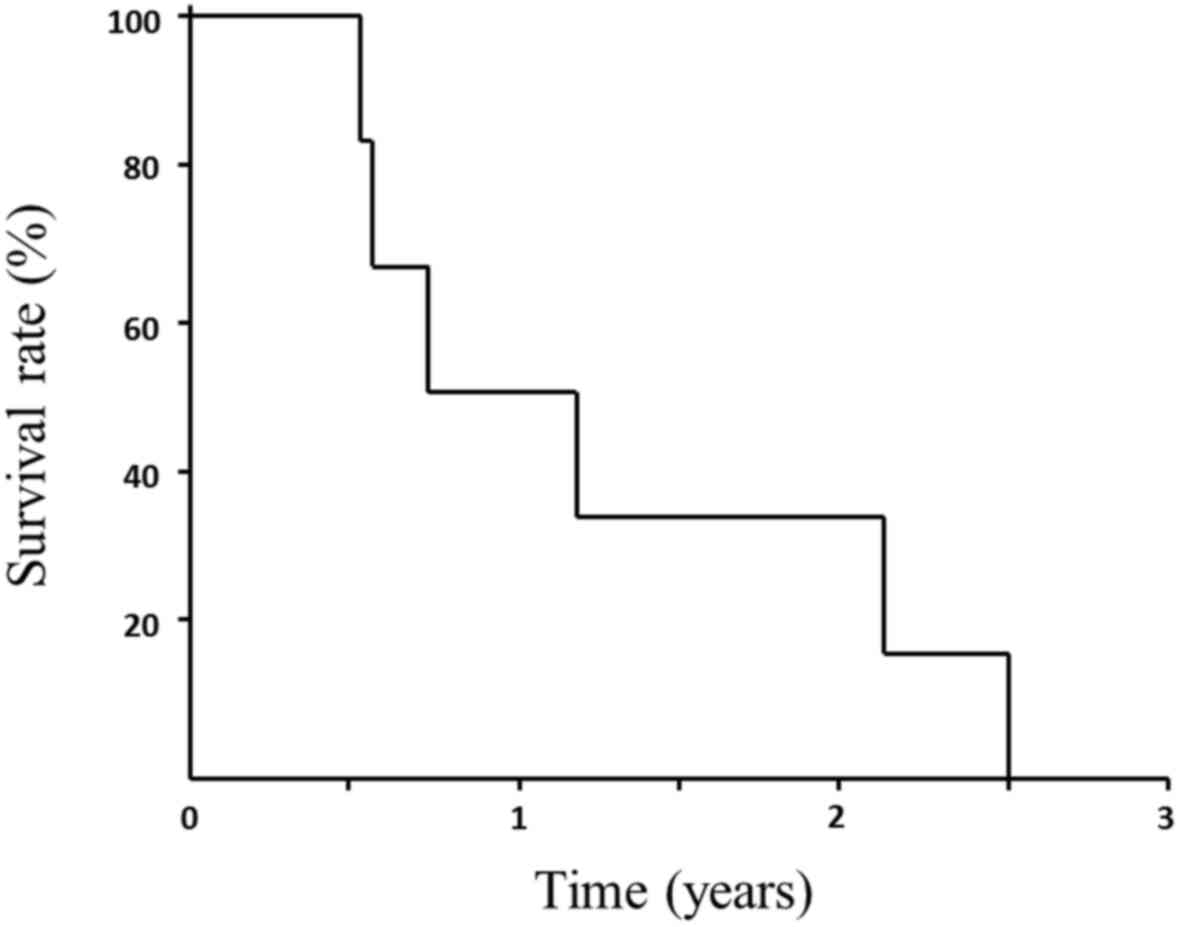
The OS and MST from treatment initiation until death
were 15.4 (range, 6.5–31.1) and 9.0 months, respectively (Fig. 1).
Discussion
The prognosis of patients with unresectable and
recurrent BTC is very poor, and standard chemotherapy for patients
with unresectable BTC was not available until the discovery of the
increased efficacy of cisplatin plus GEM cited above (2). However, the median OS of patients
treated with cisplatin plus GEM was reported to be only 11.7 months
(2). 5-FU is a major drug used to
treat hepatobiliary and pancreatic cancers; however, phase II
studies of combinations primarily based on 5-FU regimens show
little or no benefit in terms of survival and quality of life
(11,12). S-1 is an oral prodrug of 5-FU that is
widely used in Japan. In the phase III GEST trial, S-1 achieved a
favorable response and was not inferior to GEM in increasing the OS
of patients with unresectable pancreatic cancer (13). Moreover, the JASPAC 01 trial found
that S-1 prior to GEM is effective as adjuvant chemotherapy for
resected pancreatic cancer (14).
However, there is no available third-line chemotherapy following
GEM (plus cisplatin) and 5-FU for the treatment of patients with
BTC.
PTX is isolated from the Western Yew, Taxus
brevifolia (3). Similar to vinca
alkaloids, PTX binds microtubules. However, while vinca alkaloids
promote microtubule dissociation and disruption of the mitotic
spindle, PTX promotes microtubule formation and stabilization.
Retrospective studies, as well as phase I and II studies of PTX and
docetaxel (taxanes) for the treatment of patients with pancreatic
cancer and BTC (15–19), reported disease control rates of
33–57% when this regimen was used as first-line chemotherapy.
PTX is attracting increasing attention for its
effects on pathological conditions other than cancer. For example,
PTX is incorporated into drug-eluting stents placed in coronary
arteries (20). Moreover, PTX
ameliorates fibrosis in hepatic stellate cells and renal fibrosis
through inhibition of TGF-β/Smad activity (6,7).
Furthermore, PTX inhibits paracrine TGF-β1 signaling between
gallbladder epithelial cells and myofibroblasts (21).
PTX decreases interstitial fluid pressure and
improves the oxygenation of breast cancer tissues in patients
treated with neoadjuvant chemotherapy (22). To mitigate this problem, patients
with hypoxic tumors, tumors with high interstitial fluid pressure,
or both, were administered PTX chemotherapy (22). Thus, taxanes may be effective in
treating hypoxic tumors, such as pancreatic cancer and BTC. For
example, albumin-bound PTX + GEM therapy increases the treatment
options for patients with pancreatic cancer (23), and this therapy causes stromal
disrupting effects in these patients (24).
Taxane chemotherapy has been used to treat patients
with GEM-refractory pancreatic cancer (25,26).
Anticancer drugs, irradiation, hypoxia, malnutrition and heat
induce EMT, which is associated with the invasive potential of
cancer cells (27). The inhibitory
effect of PTX on TGF-β/Smad activity contributes to the suppression
of the EMT (8).
We previously reported that PTX was more effective
in terms of time-to-treatment failure compared with GEM and S-1 for
the treatment a patient with unresectable gallbladder cancer
(9). Furthermore, low-dose PTX,
which is associated with fewer side effects, should be used as
palliative chemotherapy for patients with BTC; in addition, PTX was
established as a palliative chemotherapy agent for treating
patients with breast cancer (28).
In the present phase I study, the efficacy and safety of weekly
low-dose PTX as third-line chemotherapy for patients with BTC was
demonstrated. The 50 mg/m2 of PTX was not MTD after 8
weeks of administration; however, a further dose increment, which
would be required to treat patients with RD, was not pursued. The
intent of this therapy was palliative, aimed to be administered for
as long as possible, safe and painless; therefore, the appropriate
dose of PTX was terminated at 50 mg/m2. This dose may be
referred to as minimum effective dose and it may be applied in
palliative treatment.
In conclusion, following failure of therapy with GEM
and 5-FU, palliative chemotherapy with low-dose PTX was found to be
well-tolerated and safe, and may be effective for patients with
unresectable or recurrent BTC. A future phase II study is required
to confirm the effectiveness of low-dose PTX in this setting.
References
|
1
|
Xiu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valle JW, Wasan H, Palmer DH, Cunningham
D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S,
Pereira SP, et al: Cisplatin plus gemcitabine versus gemcitabine
for biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelmon K: The toxoids: Paclitaxel and
docetaxel. Lancet. 344:1267–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donaldson KL, Goolsby GL, Kiener PA and
Wahl AF: Activation of p34cdc2 coincident with taxol-induced
apoptosis. Cell Growth Differ. 5:1041–1050. 1994.PubMed/NCBI
|
|
5
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang D, Sun L, Xian W, Liu F, Ling G,
Xiao L, Liu Y, Peng Y, Haruna Y and Kanwar YS: Low-dose paclitaxel
ameliorates renal fibrosis in rat UUO model by inhibition of
TGF-beta/Smad activity. Lab Invest. 90:436–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou J, Zhong DW, Wang QW, Miao XY and Xu
XD: Paclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta/Smad activity. World J Gastroenterol.
16:3330–3334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirose A, Tajima H, Ohta T, Tsukada T,
Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H,
et al: Low-dose paclitaxel inhibits the induction of
epithelial-mesenchymal transition in the human cholangiocarcinoma
CCKS-1 cell line. Oncol Lett. 6:915–920. 2013.PubMed/NCBI
|
|
9
|
Tajima H, Ohta T, Shinbashi H, Hirose A,
Tsukada T, Okamoto K, Nakanuma S, Sakai S, Furukawa H, Makino I, et
al: Successful treatment of unresectable gallbladder cancer with
low-dose paclitaxel as palliative chemotherapy after failure of
gemcitabine and oral S-1: A case report. Oncol Lett. 4:1281–1284.
2012.PubMed/NCBI
|
|
10
|
Eienhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellis PA, Norman A, Hill A, O'Brien ME,
Nicolson M, Hickish T and Cunningham D: Epirubicin, cisplatin and
infusional 5-fluoroufacil (5-FU) (ECF) in hepatobiliary tumors. Eur
J Cancer. 31A:1594–1598. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patt YZ, Jones DV Jr..Hoque A, Lozano R,
Markowitz A, Raijman I, Lynch P and Charnsangavej C: Phase II trial
of intravenous fluorouracil and subcutaneous interferon alfa-2b for
biliary tract cancer. J Clin Oncol. 14:2311–2315. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomized,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papakostal P, Kouroussis C, Androulakis N,
Samelis G, Aravantinos G, Kalbakis K, Sarra E, Souglakos J,
Kakolyris S and Georgoulias V: First-line chemotherapy with
docetaxel for unresectable or metastatic carcinoma of the biliary
tract. A multicentre phase II study. Eur J Cancer. 37:1833–1838.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada S, Sakata Y, Matsuno S, Kurihara M,
Sasaki Y, Ohashi Y and Taguchi T: Phase II study of docetacel in
patients with metastatic pancreatic cancer: A Japanese cooperative
study. Cooperative Group of Docetaxel for Pancreatic Cancer in
Japan. Br J Cancer. 80:438–443. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryan DP, Kulke MH, Fuchs CS, Grossbard ML,
Grossman SR, Morgan JA, Earle CC, Shivdasani R, Kim H, Mayer RJ and
Clark JW: A Phase II study of gemcitabine and docetaxel in patients
with metastatic pancreatic carcinoma. Cancer. 94:97–103. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones DV Jr..Lozano R, Hoque A, Markowitz
A and Patt YZ: Phase II study of paclitaxel therapy for
unresectable biliary tree carcinomas. J Clin Oncol. 14:2306–2310.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeda S, Motoi F, Onogawa T, Morikawa T,
Shigeru O, Sakata N, Takadate T, Naitoh T, Rikiyama T, Katayose Y,
et al: Paclitaxel as second-line chemotherapy in patients with
gemcitabine-refractory pancreatic cancer: A retrospective study.
Int J Clin Oncol. 16:539–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beak I, Bai CZ, Hwang J, Nam HY, Park JS
and Kim DJ: Paclitaxel coating of the luminal surface of
hemodialysis grafts with effective suppression of neointimal
hyperplasia. J Vasc Surg. 55:806–814.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi HS, Savard CE, Choi JW, Kuver R and
Lee SP: Paclitaxel interrupts TGF-beta1 signaling between
gallbladder epithelial cells and myofibroblasts. J Surg Res.
141:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taghian AG, Abi-Raad R, Assaad SI, Casty
A, Ancukiewicz M, Yeh E, Molokhia P, Attia K, Sullivan T, Kuter I,
et al: Paclitaxel decreases the interstitial fluid pressure and
improve oxygenation in breast cancer in patients treated with
neoadjuvant chemotherapy: Clinical implications. J Clin Oncol.
23:1951–1961. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Von Hoff DD, Ramanthan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muňoz M, Quijano Y, Cubillo A,
Rodoriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shukuya T, Yasui H, Boku N, Onozawa Y,
Fukutomi A, Yamazaki K, Taku K, Kojima T and Machida N: Weekly
paclitaxel after failure of gemcitabine in pancreatic cancer
patients with malignant ascites: A retrospective study. Jpn J Clin
Oncol. 40:1135–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cereda S and Reni M: Weekly docetaxel as
salvage therapy in patients with gemcitabine-refractory metastatic
pancreatic cancer. J Chem. 20:509–512. 2008.
|
|
27
|
Tajima H, Ohta T, Makino I, Hayashi H,
Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa H, Fushida
S, et al: Expression of epithelial-mesenchymal transition markers
in locally recurrent hepatocellular carcinoma after radiofrequency
ablation. Exp Therap Med. 1:347–350. 2010.
|
|
28
|
Schrama JG, de Boer MM, Baars JW,
Schornagel JH and Rodenhuis S: Palliative chemotherapy after
failure of high-dose chemotherapy in breast cancer-toxicity and
efficacy. Anticancer Res. 23:2795–2800. 2003.PubMed/NCBI
|