Introduction
Renal cell carcinoma (RCC) accounts for 2% of all
malignant tumors worldwide, with clear-cell RCC (ccRCC) being the
most common subtype. Conventional cytotoxic drugs and radiation
therapy have demonstrated low efficacy in the treatment of RCC
(1). However, the development of an
improved understanding of the molecular basis of renal cell
carcinogenesis has permitted the development of multikinase
inhibitors that predominantly target angiogenesis (2). In patients with a high risk or
intermediate risk according to Memorial Sloan Kettering Cancer
Center (MSKCC) criteria (3), or in
patients with a symptomatic primary lesion, advanced metastatic
disease may be managed with cytoreductive nephrectomy in operable
patients, and subsequent first-line therapy with multi-targeted
tyrosine-kinase inhibitors (TKIs) that block vascular endothelial
growth factor receptor (VEGFR)-mediated cellular signaling
pathways, such as sunitinib or pazopanib (4–6). These
latest drugs provide a median overall survival time of 28.3 months
for patients treated with pazopanib, and 29.1 months for those
treated with sunitinib, with 20 or 24% of patients remaining alive
at 5 years follow-up, respectively (7).
Multi-targeted TKIs that are employed in RCC share a
class of adverse effects associated mainly with inhibition of the
VEGFR, consisting predominantly of hypertension, arterial
thromboembolic events, cardiomyopathy, hemorrhage, wound healing
complications, proteinuria, gastrointestinal perforation, hand-foot
syndrome, and diarrhea. To date, acute pancreatitis has been
reported as a rare adverse event associated with VEGFR TKIs
(8). A recent meta-analysis
evaluating the risk of pancreatitis in patients treated with
multi-targeted TKIs versus non-TKI arms in randomized clinical
trials (RCTs) demonstrated that subjects in the TKI group were at
higher risk of suffering from acute pancreatitis (8). However, the majority of cases were
attributed to the use of sunitinib, and only one case occurred in a
patient with non-small cell lung cancer (NSCLC) treated with the
combination of pazopanib and pemetrexed (8). Increases in the levels of asymptomatic
lipase and amylase associated with the use of pazopanib have also
been observed (9). Nevertheless, to
the best of our knowledge, asymptomatic acute pancreatitis has
never been reported associated with the use of pazopanib in
monotherapy in patients with RCC. In the present study, to the best
of our knowledge for the first time in the literature, a case of a
67-year-old man with metastatic RCC who developed progressive acute
pancreatitis during first-line, single-agent treatment with
pazopanib, which was resolved after discontinuation of the
treatment, is reported.
Case report
The case of a 67-year-old male patient diagnosed
with a right RCC with splenic, bilateral lung and left adrenal
metastases is reported. The patient's past medical history revealed
chronic obstructive pulmonary disease (COPD), obstructive sleep
apnea (OSA), thalassemia minor and previous polypectomy of three
tubulovillous colon polyps. At the outset, the patient was
prescribed treatment for COPD with roflumilast, fluticasone
propionate, tiotropium bromide, inhaled budesonide and terbutaline
on demand, and continuous positive airway pressure for OSA. Prior
to starting treatment, the patient had none of the major risk
factors associated with pancreatitis: There was no current habit of
smoking or alcoholism, no dyslipidemia, diabetes mellitus or
hypercalcemia, no previous history of cholelithiasis,
biliary-pancreatic infections or abdominal surgery, and prior
radiological studies had excluded the presence of cysts or
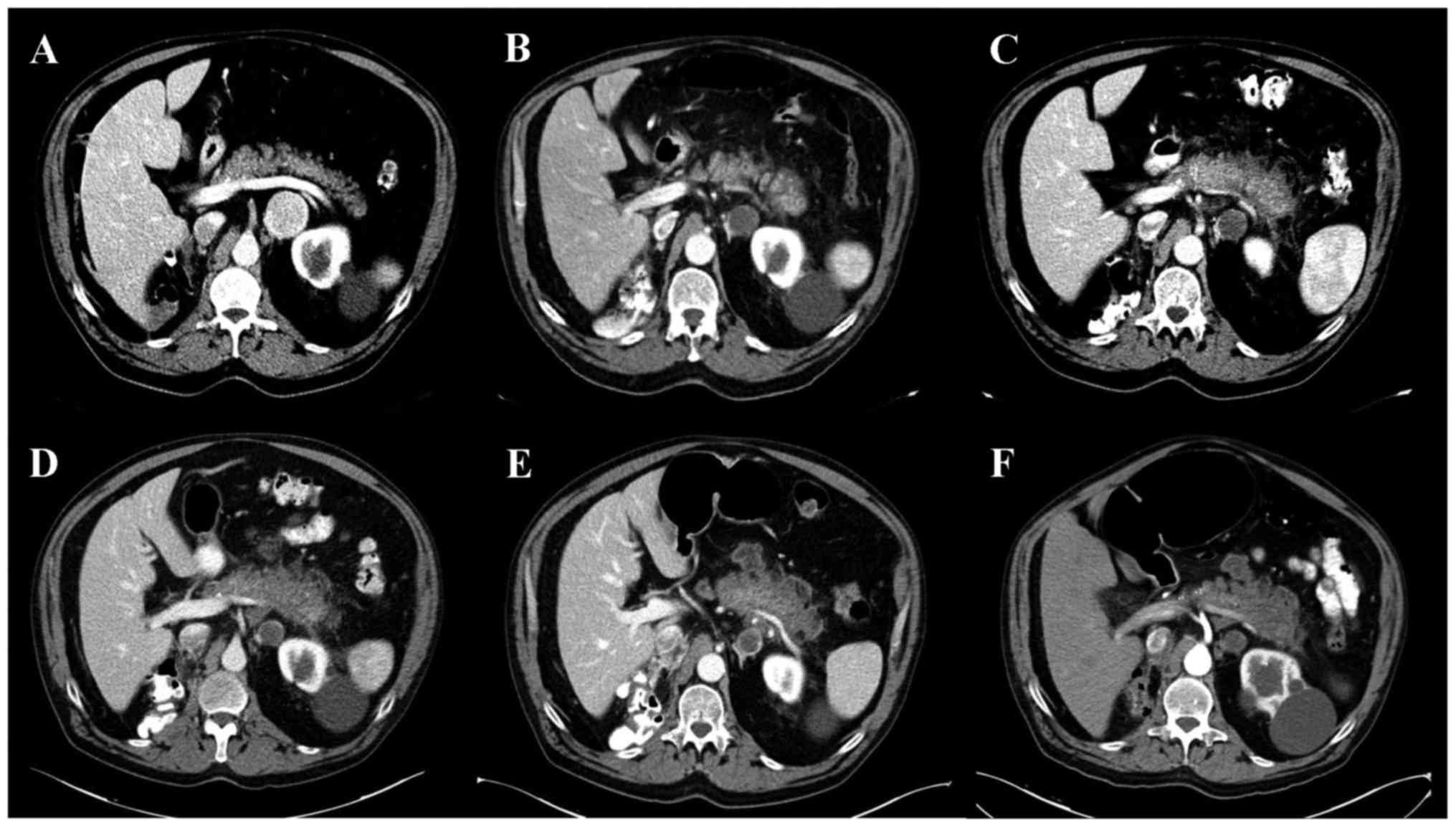
pancreatic metastasis (Fig. 1A).
After having been diagnosed with a right renal mass,
the patient underwent a right radical nephrectomy with a
confirmatory pathology report of Furhman grade II ccRCC (pT3b N0
M1; stage IV). At 4 weeks after recovery from surgery, the patient
initiated treatment with first-line pazopanib (800 mg administered
daily). At 2 months following the initiation of treatment, the
patient consulted for hand-foot syndrome in his hands (grade 1) and
feet (grade 2), according to the National Cancer Institute's Common
Terminology Criteria for Adverse Events (CTCAE v4.03). At this
stage, the blood test revealed grade 2 hypertransaminasemia and
grade 1 amylase elevation (113 mg/dl; Fig. 2). Blood analysis revealed a glucose
level of 133 mg/dl, and the level of lipase was within its normal
range. The patient denied having any of the symptoms of acute
pancreatitis or liver dysfunction. The computerized tomography (CT)
scan revealed a focal acute edematous pancreatic tail and body
pancreatitis, as well as fat striation, compatible with acute
pancreatitis with a Balthazar Score of grade C (Fig. 1B). A partial response of the
patient's metastatic disease was also observed. Due to the complete
absence of signs and symptoms of a clinically meaningful acute
pancreatitis, and considering the important clinical and
radiological benefits that had been obtained since the initiation
of pazopanib, a course of subsequent continuation of treatment was
decided.
In the following radiological evaluation, given a
marked worsening of the patient's condition, as noted in the
radiological images (Fig. 1C) and
the serum analysis of pancreatitis (Fig.
2), treatment with pazopanib was discontinued. After three more
weeks, the patient underwent a further clinical and radiographical
evaluation. An abdominal CT scan revealed stable acute pancreatitis
(Fig. 1D), although the tumor
disease was now characterized by loco-regional progression of the
splenic and left adrenal metastases. An improvement in the amylase
and transaminases levels was observed (Fig. 2). For that reason, it was decided to
resume active treatment. Considering the clinical and radiological
benefits initially obtained from the VEGF TKI treatment, a
second-line treatment was initiated, and treatment with sorafenib
(400 mg twice daily) was decided.
At 10 days after resuming treatment, the patient
experienced an incapability to walk due to grade 3 hand-foot
syndrome, and so a dose reduction to 200 mg sorafenib, to be
administered twice a day, was decided upon. Topical treatment for
the skin lesions with a mixture of retinoic acid, triamcinolone
acetonide, urea and propylene glycol was started, with consequent
amelioration, allowing deambulation. During follow up,
stabilization of the signs of pancreatitis upon radiological and
analytic examination was identified (Figs. 1E and 2). The last CT scan performed two months
after sorafenib initiation revealed retroperitoneal and liver
metastatic progression, with no changes in the appearance of the
pancreas observed on performing radiology (Fig. 1F). The patient maintained grade 1
hand-foot syndrome, with no other toxicities of grade >2
associated with sorafenib, and therefore the dose administered was
scaled up to the full dose (400 mg twice a day).
After three more months, during the last follow-up
visit, the patient remained on sorafenib and presented stable
metastatic disease, with no radiological changes in pancreatic
features and with mild skin toxicity.
The patient gave his informed consent authorizing
the publication of this case report.
Discussion
In the present study, the case of a male patient
with metastatic RCC who developed acute pancreatitis that was
diagnosed as an asymptomatic finding during his first CT scan
evaluation, two months after pazopanib initiation, was
described.
The efficacy of pazopanib is similar to that of
other TKIs used as first-line treatment in metastatic RCC, such as
sunitinib (10). The toxicity
profile of pazopanib in patients with advanced RCC accounts for
hand-foot syndrome, rash, stomatitis, dysgeusia, dyspepsia,
anorexia, nausea, vomiting, diarrhea, fatigue, weight loss, hair
color change, alopecia, hypertension, increased mean arterial blood
pressure, and liver function abnormalities (11). No evidence of clinically important
differences in the quality of life when comparing pazopanib with a
placebo has been identified (12).
Furthermore, a randomized, controlled, double-blind cross-over
trial of pazopanib versus sunitinib demonstrated patients'
preference for pazopanib over sunitinib (13).
Santoni et al (14) published a systemic review and
meta-analysis of gastrointestinal events in patients with solid
tumors who received sorafenib, sunitinib and pazopanib, including
RCC, NSCLC, hepatocellular carcinoma, breast cancer, neuroendocrine
tumors, gastrointestinal stromal tumors and soft tissue sarcomas
(14). These authors described
anorexia, diarrhea and nausea as the main gastrointestinal adverse
side-effects observed, but no cases of acute pancreatitis were
observed. By contrast, a meta-analysis evaluating the risk of
specifically developing acute pancreatitis associated with
multi-targeted TKIs has recently been published (8). In that study, phase II and phase III
RCTs in patients with different tumor origins comparing arms with
TKIs versus non-TKI treatment were included. The incidence of
pancreatitis in the two groups was specifically analyzed. A
significantly higher risk of pancreatitis was observed in the TKI
group (25 of 5,569 patients) compared with the control group (7 of
5,009 patients). The majority of the cases occurred in patients on
sunitinib. Only one patient of the 5,569 in the TKI arm, receiving
treatment with pazopanib, developed pancreatitis. However, that
subject was diagnosed with advanced NSLC, and received a
combination regimen with pemetrexed and pazopanib, so it remains
unclear whether acute pancreatitis was induced by pazopanib, by
pemetrexed, or by both drugs in combination (8). A case of isolated amylase and lipase
level elevation associated with the use of pazopanib in a patient
with metastatic RCC was also reported; however, that patient
exhibited neither symptoms nor radiological signs of pancreatitis
(9).
The toxicity shown by pazopanib when used to treat
sarcomas has been further investigated by the European Organization
for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma
Group (EORTC study 62043), with cardiovascular, gastrointestinal
and hepatic disorders, myelosuppression and proteinuria being the
major adverse effects (15). The
occurrence of pneumothorax, heart failure, venous thrombosis,
pulmonary embolism and hypothyroidism is rare, although these are
potentially serious adverse effects also associated with pazopanib
treatment (16). A case of
pazopanib-induced acute pancreatitis in a patient with cutaneous
angiosarcoma was also recently reported (17). However, no previous reports of
pancreatitis, when pazopanib has been administered as single agent,
have been reported for patients diagnosed with RCC.
To the best of our knowledge, the present study has
described the first case of development of asymptomatic acute
pancreatitis associated with a single agent, pazopanib, in a
patient with metastatic RCC. Previous radiological studies did not
reveal any disorder in the patient's pancreas prior to pazopanib
administration. However, a radiologically evident, but clinically
asymptomatic, acute pancreatitis 2 months after initiation of
pazopanib treatment was apparent. The condition was confirmed by
serum analysis (i.e. an elevation in the amylase level), and the
continuation of pazopanib following the initial diagnosis of
pancreatitis was associated with the worsening of radiological
pancreatitis. Resolution of the inflammation was achieved by
withholding the medication, revealing a temporal and
dose-accumulation association between both factors (acute
pancreatitis and pazopanib treatment). The presumed
pathophysiological mechanism for this rare adverse effect would
include the ability of pazopanib (and, potentially, of other VEGFR
TKIs) to affect the pancreatic cells, or, more likely, the vascular
endothelial receptors in the vessels of the pancreas, causing
ischemia of the pancreatic tissue (18).
Given the fact that asymptomatic pancreatitis is a
rare example of toxicity associated with pazopanib and a follow-up
CT scan is performed every 8–12 weeks, the present authors suggest
monitoring amylase and lipase levels at baseline and during
treatment with pazopanib and other antiangiogenic TKIs. In those
patients who show increasing levels of acute
pancreatitis-associated enzymes, an abdominal CT scan should be
performed in order to rule out rapidly progressing acute
pancreatitis. According to the present case study, switching to a
different TKI may be feasible as an option, since it seems to be
more likely to be a specific drug-associated, rather than a
class-associated event.
As shown in the present case report, multi-targeted
VEGFR TKIs are the standard of care against advanced RCC, since the
administration of these drugs is capable of resulting in a marked
change in the life expectation of patients with this disease, with
a median overall survival of ~28–30 months for patients treated
with pazopanib and sunitinib. However, VEGFR TKIs are also
associated with rare, but potentially life-threatening, adverse
events, such as hemorrhage and cardiovascular events. Although
acute pancreatitis has not been a commonly suspected toxicity in
patients receiving pazopanib, evidence that has shown the
possibility of developing this condition with sorafenib, and the
findings of the present case study following pazopanib treatment
may change the way in which patients on these drugs are monitored,
and perhaps serum markers of pancreatitis may be added to routine
clinical practice. In case of confirming acute pancreatitis
associated with a particular drug, switching to a different VEGFR
TKI may maintain the clinical benefits, leading to an improvement
in, or maybe even resolving, the pancreatitis.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
TKIs
|
tyrosine-kinase inhibitors (TKIs)
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
CT
|
computerized tomography
|
|
RCT
|
randomized clinical trial
|
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baldewijns MM, van Vlodrop IJH, Vermeulen
PB, Soetekouw PM, van Engeland M and de Bruïne AP: VHL and HIF
signalling in renal cell carcinogenesis. J Pathol. 221:125–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Mazumdar M, Bacik J, Berg W,
Amsterdam A and Ferrara J: Survival and prognostic stratification
of 670 patients with advanced renal cell carcinoma. J Clin Oncol.
17:2530–2540. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B, Eisen T, Porta C, Patard JJ,
Khoo V, Algaba F, Mulders P and Kataja V: ESMO Guidelines Working
Group: Renal cell carcinoma: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 23:(Suppl 7).
vii65–vii71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Agarwal N, Beard C, Bolger GB,
Boston B, Carducci MA, Choueiri TK, Figlin RA, Fishman M, Hancock
SL, et al: NCCN clinical practice guidelines in oncology: Kidney
cancer. J Natl Compr Canc Netw. 7:618–630. 2009.PubMed/NCBI
|
|
6
|
Bellmunt J, Puente J, de Garcia Muro J,
Lainez N, Rodríguez C and Duran I: Spanish Society for Medical
Oncology: SEOM clinical guidelines for the treatment of renal cell
carcinoma. Clin Transl Oncol. 16:1043–1050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motzer RJ, Hutson TE, McCann L, Deen K and
Choueiri TK: Overall survival in renal-cell carcinoma with
pazopanib versus sunitinib. N Engl J Med. 370:1769–1770. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghatalia P, Morgan CJ, Choueiri TK, Rocha
P, Naik G and Sonpavde G: Pancreatitis with vascular endothelial
growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol
Hematol. 94:136–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russano M, Vincenzi B, Venditti O,
D'Onofrio L, Ratta R, Guida FM, Tonini G and Santini D: Pazopanib
and pancreatic toxicity: A case report. BMC Res Notes. 8:1962015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suttle AB, Ball HA, Molimard M, Hutson TE,
Carpenter C, Rajagopalan D, Lin Y, Swann S, Amado R and Pandite L:
Relationships between pazopanib exposure and clinical safety and
efficacy in patients with advanced renal cell carcinoma. Br J
Cancer. 111:1909–1916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Escudier B, Porta C, Bono P, Powles T,
Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins
R, et al: Randomized, controlled, double-blind, cross-over trial
assessing treatment preference for pazopanib versus sunitinib in
patients with metastatic renal cell carcinoma: PISCES Study. J Clin
Oncol. 32:1412–1418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santoni M, Conti A, De Giorgi U, Iacovelli
R, Pantano F, Burattini L, Muzzonigro G, Berardi R, Santini D and
Cascinu S: Risk of gastrointestinal events with sorafenib,
sunitinib and pazopanib in patients with solid tumors: A systematic
review and meta-analysis of clinical trials. Int J Cancer.
135:763–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sleijfer S, Ray-Coquard I, Papai Z, Le
Cesne A, Scurr M, Schöffski P, Collin F, Pandite L, Marreaud S, De
Brauwer A, et al: Pazopanib, a multikinase angiogenesis inhibitor,
in patients with relapsed or refractory advanced soft tissue
sarcoma: A phase II study from the European organisation for
research and treatment of cancer-soft tissue and bone sarcoma group
(EORTC study 62043). J Clin Oncol. 27:3126–3132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawakubo K, Hata H, Kawakami H, Kuwatani
M, Kawahata S, Kubo K, Imafuku K, Kitamura S and Sakamoto N:
Pazopanib-Induced Severe Acute Pancreatitis. Case Rep Oncol.
8:356–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amar S, Wu KJ and Tan WW:
Sorafenib-induced pancreatitis. Mayo Clin Proc. 82:5212007.
View Article : Google Scholar : PubMed/NCBI
|