Introduction
Pancreatobiliary tract cancer is a general term for
malignancies in the pancreas, gallbladder and extrahepatic bile
ducts. Despite advances in cancer treatment, the prognosis of
pancreatobiliary tract cancer remains very poor (1,2).
According to the American Cancer Society (3), for all stages of pancreatic cancer
combined, the 1-year relative survival rate is 20%, and the 5-year
rate is 6%. The risk factors for pancreatic cancer include old age,
male gender, smoking, chronic pancreatitis, type 2 diabetes
mellitus, obesity and a family history of pancreatic cancer
(4). However, the prognostic factors
for pancreatic cancer remain unclear. Furthermore, possibly due to
its infrequent occurrence and rapidly fatal outcome, little
clinical research has been performed on the etiology of gallbladder
and extrahepatic bile duct cancer. Therefore, it is crucial to
investigate factors affecting the survival rate of pancreatobiliary
tract cancer.
It has been reported that periodontitis is
associated with numerous systemic diseases, including cancer
(5). Periodontitis is a chronic
inflammatory disease of the tissues that support the teeth, and it
is initiated by overgrowth of anaerobic bacteria in subgingival
sites. Development of periodontitis depends on the interaction
between bacterial products, such as lipopolysaccharides, and the
host, leading to cytokine production that mediates periodontal
tissue destruction. Periodontal infections are associated with
elevated concentrations of circulating inflammatory cytokines
(6,7)
and the severity of periodontitis is directly correlated with serum
concentrations of inflammatory cytokines (8). Upregulation of inflammatory cytokine
pathways have been shown to modulate pancreatic cancer progression
(9). These studies suggest that
periodontitis may affect the progression as well as the prognosis
of pancreatobiliary tract cancer.
Previous studies have indicated that periodontitis
is a risk factor for pancreatic cancer (10,11).
However, the association between periodontitis and the prognosis of
pancreatobiliary tract cancer remains unclear, but it has been
hypothesized that severe periodontitis may affect the prognosis of
pancreatobiliary tract cancer. The aim of this pilot study was to
investigate the association between periodontitis and the prognosis
of pancreatobiliary tract cancer.
Patients and methods
Study population
A total of 77 patients were diagnosed with primary
cancer of the pancreas, bile ducts or gallbladder at the Department
of Gastroenterology and Hepatology, Okayama University Hospital
(Okayama, Japan), between July, 2013 and July, 2014. Among those
patients, 30 (20 men and 10 women) were referred to the Department
of Preventive Dentistry for oral health checks prior to cancer
therapy. Patients who were aged <50 years (1 patient), who had
<10 teeth (4 patients), whose 1-year survival data were missing
(2 patients) and who lacked data (1 patient) were excluded from
this study. As a result, data from 22 patients (14 men and 8 women)
were analyzed. This study was approved by the Ethics Committee of
the Okayama University Graduate School of Medicine, Dentistry and
Pharmaceutical Sciences and the Okayama University Hospital (no.
1506-052). Written informed consent was obtained from all patients
who agreed to participate.
Oral examination
One dentist recorded the following data: Number of
teeth present, probing pocket depth (PPD), clinical attachment
level (CAL), percentage of sites with bleeding on probing (%BOP)
and percentage of sites with plaque accumulation. PPD and CAL were
measured for all teeth present, except for third molars, using a
color-coded probe (CP-8, Hu-Friedy, Chicago, IL, USA). CAL was
measured as the distance between the cemento-enamel junction and
the base of the periodontal pocket. PPD and CAL were measured at
six sites (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual,
mid-lingual and disto-lingual). BOP was defined as the presence of
bleeding after gentle probing with 25 g probing force. Plaque level
was measured after staining with erythrosine and was recorded as
the presence or absence of plaque at four sites (mesial, distal,
buccal and lingual) around each tooth. Mean PPD and CAL, %BOP and
plaque level were calculated for each patient. In addition, the
severity of periodontitis (no, mild, moderate or severe
periodontitis) for each patient was defined as previously described
(12).
General status examination
Prior to cancer therapy, medical charts were
reviewed to obtain information on cancer site, cancer stage, body
weight and height. Body mass index (BMI) was calculated using body
weight and height data. A personal interview was conducted to
obtain information on smoking habits (pack-years). In addition,
biochemical serum markers, including hemoglobin A1c (HbA1c),
C-reactive protein (CRP), albumin, carcinoembryonic antigen (CEA)
and carbohydrate antigen 19-9 (CA19-9) were also evaluated. These
serum markers are commonly assessed in the prognosis of
pancreatobiliary tract cancer (13).
Serum HbA1c concentration was measured by high-performance liquid
chromatography. Serum CRP concentration was measured using the
latex agglutination method. Serum albumin concentration was
measured by the bromocresol green albumin method. Serum CEA and
CA19-9 concentrations were measured by an electrochemiluminescene
immunoassay. Furthermore, therapeutic approach and the occurrence
of cholangitis during cancer treatment were recorded. Treatment
plans were determined by standard protocols according to the tumor
stage and physical condition of the patient.
Statistical analysis
The patients in this study were divided into two
groups according to 1-year survival, namely those surviving for
<1 year (n=11, 7 men and 4 women) and those surviving for ≥1
year (n=11, 7 men and 4 women). Means ± standard deviation in both
groups were calculated for continuous variables: Age, BMI, smoking
habit (pack-years), number of teeth present, mean PPD, mean CAL,
%BOP, plaque level, and concentrations of HbA1c, CRP, albumin, CEA
and CA19-9. Numbers and percentages are presented for categorical
variables: Gender, cancer site, cancer stage, presence of
chemotherapy, presence of cholangitis and severity of
periodontitis. The Mann-Whitney U test and Fisher's exact test were
used to assess significant differences in clinical variables
between the two groups. Median survival was estimated using the
Kaplan-Meier method and differences were tested using the log-rank
test. The follow-up period was 1 year after diagnosis. Backward
stepwise regression procedures for the Cox proportional hazards
model were used to evaluate significant prognostic factors. In the
Cox proportional hazards model, the following 7 candidate variables
were selected: Age, gender, BMI, serum concentration of HbA1c,
CA19-9 and CRP, and severity of periodontitis. P<0.05 was
considered to indicate statistically significant differences. All
the analyses were performed using the SPSS 15.0 J software program
for Windows (IBM Japan, Tokyo, Japan).
Results
Patient characteristics
The characteristics of all the patients are
summarized in Table I. Significant
differences between the <1-year and the ≥1-year survival groups
were observed in serum CRP concentration (P=0.013), serum CEA
concentration (P=0.028), mean CAL (P=0.031) and severity of
periodontitis (P=0.033). There were no significant differences in
the other variables between the two groups.
 | Table I.Differences in clinical parameters
between the <1-year and ≥1-year survival groups. |
Table I.
Differences in clinical parameters
between the <1-year and ≥1-year survival groups.
| Variables | Category | Total (n=22) | ≥1 year survival
(n=11) | <1 year survival
(n=11) | P-value |
|---|
| Age (years) |
| 68.7±9.0 | 68.4±10.5 | 69.1±7.6 | 0.855a |
| Gender | Male | 14 (63.6) | 7 (63.6) | 7 (63.6) | 1.000b |
| BMI
(kg/m2) | <18.5 | 2 (9.1) | 0 (0.0) | 2 (18.2) | 0.329c |
|
| 18.5–24.9 | 18 (81.8) | 10 (90.9) | 8 (72.7) |
|
|
| ≥25.0 | 2 (9.1) | 1 (9.1) | 1 (9.1) |
|
| Primary site | Pancreas | 18 (81.8) | 9 (81.8) | 9 (81.8) | 1.000b |
|
|
Gallbladder/extrahepatic bile duct | 4 (18.2) | 2 (18.2) | 2 (18.2) |
|
| Cancer stage | I | 1 (4.5) | 1 (9.1) | 0 (0.0) | 0.349c |
|
| II | 1 (4.5) | 1 (9.1) | 0 (0.0) |
|
|
| III | 3 (13.6) | 2 (18.2) | 1 (9.1) |
|
|
| IVa | 9 (40.9) | 5 (45.5) | 4 (36.4) |
|
|
| IVb | 8 (36.4) | 2 (18.2) | 6 (54.5) |
|
| Chemotherapy | Yes | 19 (86.4) | 9 (81.8) | 10 (90.9) | 0.500b |
| Cholangitis | Present | 1 (4.5) | 0 (0.0) | 1 (9.1) | 0.500b |
| Smoking status |
| 18.0 | 29.3 | 0.0 | 0.438d |
| (pack-years) |
| (0.0,39.0) | (0.0,39.0) | (0.0,38.0) |
| HbA1c (%) |
| 6.0 | 5.9 | 6.0 | 0.519d |
|
|
| (5.5,6.2) | (5.5,6.1) | (5.5,6.3) |
| CRP (mg/dl) |
| 0.23 | 0.15 | 0.81 | 0.013d |
|
|
| (0.08,0.84) | (0.07,0.23) | (0.15,1.54) |
| Albumin (g/dl) |
| 3.8 | 4.0 | 3.6 | 0.243d |
|
|
| (3.5,4.3) | (3.7,4.3) | (3.4,4.0) |
| CEA (ng/ml) |
| 6.5 | 4.6 | 13.3 | 0.028d |
|
|
| (2.6,15.2) | (2.1,8.3) | (6.0,82.3) |
| CA19-9 (U/ml) |
| 216 | 184 | 183 | 0.853d |
|
|
| (52,801) | (11,443) | (47,1748) |
| Number of teeth
present |
| 22.6±6.0 | 24.3±5.2 | 20.9±6.5 | 0.195a |
| Mean PPD (mm) |
| 2.06±0.50 | 1.93±0.38 | 2.18±0.59 | 0.259a |
| Mean CAL (mm) |
| 2.80±1.01 | 2.33±0.55 | 3.26±1.16 | 0.031 a |
| BOP (%) |
| 10.4±12.3 | 7.4±6.6 | 13.5±16.0 | 0.251a |
| Plaque level
(%) |
| 31.9±23.2 | 26.9±22.0 | 36.9±24.4 | 0.323a |
| Severity of | No/mild/ | 11 (50.0) | 8 (72.7) | 3 (27.3) | 0.033c |
| periodontitis | moderate |
|
|
| Severe | 11 (50.0) | 3 (27.3) | 8 (72.7) |
Multivariate analysis of prognostic
factors
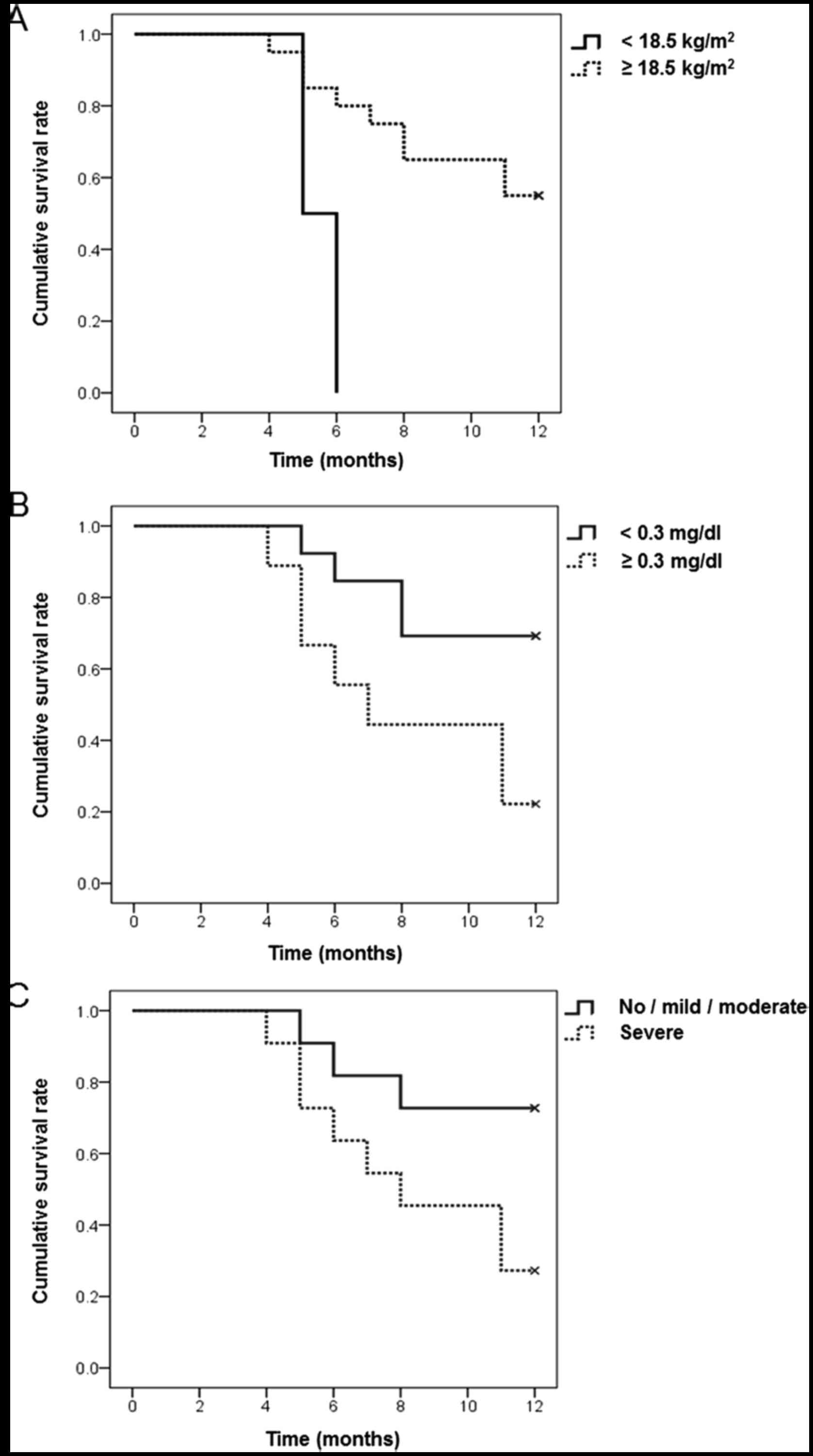
Kaplan-Meier analyses using log-rank tests revealed
that BMI, serum CRP concentration and severity of periodontitis
were significant prognostic factors for survival rate (Fig. 1). However, the Cox proportional
hazards regression model demonstrated that serum CA19-9 and CRP
concentrations were significantly associated with the prognosis of
pancreatobiliary tract cancer (Table
II).
 | Table II.Multivariate analysis of prognostic
factors. |
Table II.
Multivariate analysis of prognostic
factors.
| Factors | Hazard ratio | 95% CI |
P-valuea |
|---|
| Age | 0.87 | 0.75–1.00 | 0.060 |
| HbA1c (%) | 1.96 | 0.96–3.98 | 0.064 |
| CA19-9 (U/ml) | 1.002 | 1.000–1.004 | 0.030 |
| CRP (mg/dl) | 2.57 | 1.15–5.74 | 0.022 |
Comparison of CRP, albumin and HbA1c
according to the severity of periodontitis
The serum CRP concentration was significantly higher
and the serum albumin concentration was significantly lower in
patients with severe periodontitis group when compared to those
without severe periodontitis (P<0.05; Table III).
 | Table III.Comparisons of CRP, albumin, and
HbA1c according to severity of periodontitis. |
Table III.
Comparisons of CRP, albumin, and
HbA1c according to severity of periodontitis.
| Variables | No/mild/moderate
periodontitis (n=11) | Severe
periodontitis (n=11) |
P-valuea |
|---|
| CRP (mg/dl) | 0.12
(0.07,0.34) | 0.54
(0.15,1.54) | 0.034 |
| Albumin (g/dl) | 4.0 (3.8,4.3) | 3.5 (3.3,3.7) | 0.016 |
| HbA1c (%) | 6.0 (5.5,6.1) | 5.9 (5.1,7.0) | 1.000 |
Discussion
To the best of our knowledge, this is the first
study to investigate the association between the severity of
periodontitis and the prognosis of pancreatobiliary tract cancer.
In this study, the <1-year survival group exhibited higher
levels of serum CRP concentration, serum CEA concentration, CAL and
severity of periodontitis compared with the ≥1-year survival group.
In addition, the Kaplan-Meier method demonstrated that low BMI,
high serum CRP concentration and severe periodontitis were
significant prognostic factors for survival rate. These results
indicate that the prognosis of pancreatobiliary tract cancer was
associated with the severity of periodontitis, as well as other
factors, such as CRP. However, the Cox proportional hazards
regression model demonstrated that the serum CA19-9 and CRP
concentrations were significant prognostic factors, whereas the
severity of periodontitis was not. Therefore, the association
between the severity of periodontitis and the prognosis of
pancreatobiliary tract cancer may be indirect.
Serum CRP, an indicator of systemic inflammation, is
produced mainly by the liver in response to inflammation, infection
and tissue damage (14). According
to our findings, cancer patients with severe periodontitis had
higher levels of serum CRP compared with those without severe
periodontitis. This indicates that pancreatobiliary tract cancer
patients with severe periodontitis had more severe systemic
inflammation. A previous study demonstrated that the 1-year overall
survival rate and median survival time in patients with higher CRP
concentration (>0.3 mg/dl) were lower compared with those in the
patients with lower CRP concentration (≤0.3 mg/dl) (15). This suggests that periodontitis
affects the prognosis of pancreatobiliary tract cancer via the
upregulation of systemic inflammation.
Studies have reported a significant association
between periodontitis and serum CRP concentration. There is
evidence that individuals with chronic periodontitis have elevated
serum CRP concentrations when compared with periodontally healthy
controls (16). It has also been
reported that the presence of Porphyromonas gingivalis in
subgingival plaque is significantly associated with raised serum
CRP concentration (17). These
observations are consistent with the present results, which
indicate that severe periodontitis induces elevated serum CRP
concentration.
Serum albumin is also known to be an independent
predictor of survival in pancreatobiliary tract cancer (18). In our findings, serum albumin
concentration was significantly lower in patients with severe
periodontitis compared with that in patients without severe
periodontitis. This suggests that periodontitis affects the
prognosis of pancreatobiliary tract cancer by decreasing serum
albumin level. However, there were no significant differences
between the early and late survivor groups in terms of serum
albumin concentration in this study. In the present study, the
median serum albumin concentrations of the early and the late
survivor group were 3.6 and 4.0 g/dl, respectively. As serum
albumin concentration in this study was close to the reference
value (3.8–5.3 g/dl), it may have little effect on the prognosis of
pancreatobiliary tract cancer.
Several studies have reported a significant
association between periodontitis and pancreatic cancer. Chang
et al (11) reported that
periodontal disease was positively associated with pancreatic
cancer risk among patients aged ≥65 years. Another study reported
that individuals with high levels of antibodies against
Porphyromonas gingivalis ATTC 53978 had a two-fold higher
risk of pancreatic cancer compared with individuals with lower
levels of these antibodies (19).
Our results are supported by these findings indicating an
association between periodontitis and pancreatic cancer. However,
further studies are required to elucidate the mechanisms through
which periodontitis affects the prognosis of pancreatic cancer.
There are few studies in the scale of a pilot study,
although it is recommended to obtain 10% of the final study size
(20). In the present study, the
number of subjects in the <1-year and ≥1-year survival groups
was 11 each. When the final study size was calculated using the
data of the current pilot study (Table
I), 18 patients per group would have to be included to
demonstrate significant differences between cancer patients with
and those without severe periodontitis. As the present sample size
was >10% of the final study size, it is conceivable that 11
patients per group is sufficient as a pilot study.
The present study had certain limitations. First,
all the patients were recruited from the Okayama University
Hospital. This may limit the application of our findings to the
general population. Second, the application of periodontal
treatment during cancer treatment was not investigated in this
study, which may have affected the prognosis of pancreatobiliary
tract cancer.
In conclusion, pancreatobiliary tract cancer
patients with severe periodontitis had a poorer prognosis compared
with those without severe periodontitis. Severe periodontitis may
be indirectly associated with the survival rate of pancreatobiliary
tract cancer through promoting systemic inflammation.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research (26670904) from the Ministry of Education,
Culture, Sports, Science and Technology, Tokyo, Japan.
Glossary
Abbreviations
Abbreviations:
|
BMI
|
body mass index
|
|
%BOP
|
percentage of sites with bleeding on
probing
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CAL
|
clinical attachment level
|
|
CEA
|
carcinoembryonic antigen
|
|
CI
|
confidence interval
|
|
CRP
|
C-reactive protein
|
|
HbA1c
|
hemoglobin A1c
|
|
HR
|
hazard ratio
|
|
PPD
|
probing pocket depth
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
3
|
American Cancer SocietyCancer Facts &
Figures 2013. Atlanta: American Cancer Society; 2013
|
|
4
|
Adami HO and Trichopoulos D: Pancreatic
cancerEkbom A and Trichopoulos D: Textbook of Cancer Epidemiology.
2nd. Oxford University Press; New York: pp. 333–348. 2008
|
|
5
|
Fitzpatrick SG and Katz J: The association
between periodontal disease and cancer: A review of literature. J
Dent. 38:83–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joshipura KJ, Wand HC, Merchant AT and
Rimm EB: Periodontal disease and biomarkers related to
cardiovascular disease. J Dent Res. 83:151–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loos BG: Systemic markers of inflammation
in periodontitis. J Periodontol. 76:(11 Suppl). S2106–S2115. 2005.
View Article : Google Scholar
|
|
8
|
Amabile N, Susini G, Pettenati-Soubayroux
I, Bonello L, Gil JM, Argues S, Bonfil JJ and Paganelli F: Severity
of periodontal disease correlates to inflammatory systemic status
and independently predicts the presence and angiographic extent of
stable coronary artery disease. J Intern Med. 263:644–652. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roshani R, McCarthy F and Hagemann T:
Inflammatory cytokines in human pancreatic cancer. Cancer Lett.
345:157–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michaud DS, Joshipura K, Giovannucci E and
Fuchs CS: A prospective study of periodontal disease and pancreatic
cancer in US male health professionals. J Natl Cancer Inst.
99:171–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang JS, Tsai CR, Chen LT and Shan YS:
Investigating the association between periodontal disease and risk
of pancreatic cancer. Pancreas. 45:134–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eke PI, Page RC, Wei L, Thornton-Evans G
and Genco RJ: Update of the case definitions for population-based
surveillance of periodontitis. J Periodontol. 83:1449–1454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le N, Sund M and Vinci A: GEMS
collaborating group of Pancreas 2000: Prognostic and predictive
markers in pancreatic adenocarcinoma. Dig Liver Dis. 48:223–230.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishi T, Nakamura A, Itasaka S, Shibuya K,
Matsumoto S, Kanai M, Kodama Y, Takaori K, Mizowaki T and Hiraoka
M: Pretreatment C-reactive protein level predicts outcome and
patterns of failure after chemodadiotherapy for locally advanced
pancreatic cancer. Pancreatology. 15:694–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paraskevas S, Huizinga JD and Loos BG: A
systematic review and meta-analyses on C-reactive protein in
relation to periodontitis. J Clin Periodontol. 35:277–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winning L, Patterson CC, Cullen KM,
Stevenson KA, Lundy FT, Kee F and Linden GJ: The association
between subgingival periodontal pathogens and systemic
inflammation. J Clin Periodontol. 42:799–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siddiqui A, Heinzerling J, Livingston EH
and Huerta S: Predictors of early mortality in veteran patients
with pancreatic cancer. Am J Surg. 194:362–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michaud DS, Izard J, Wilhelm-Benartzi CS,
You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M,
Fedirko V, et al: Plasma antibodies to oral bacteria and risk of
pancreatic cancer in a large European prospective cohort study.
Gut. 62:1764–1770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lackey NR and Wingate AL: The pilot study:
One key to research successBrink PJ and Wood MJ: Advanced Design in
Nursing Research. 2nd. Sage Publications; CA: 1998, View Article : Google Scholar
|