Introduction
Myelodysplastic syndromes (MDS) are a group of
clonal hematological disorders characterized by ineffective
hematopoiesis, peripheral cytopenias and an increased risk of
development of acute myeloid leukemia (1). A number of chromosomal abnormalities
have been detected in ~50–60% of patients with de novo MDS
and in ≤80% of patients with therapy-related MDS (2). Among the classical oncogenic
abnormalities, those involving the tumor protein 53 (TP53) gene
have been extensively investigated in MDS. TP53 mutations were
detected primarily in high-risk and therapy-related MDS, frequently
in association with complex chromosomal abnormalities (3,4). In such
diseases, TP53 mutations were revealed to have a negative
prognostic impact (3,5).
The wild-type p53 protein is normally undetectable
by immunohistochemistry (IHC), due to its short half-life. By
contrast, the majority of mutated proteins have a prolonged
half-life, accumulate within the nucleus and can be easily detected
in formalin-fixed, paraffin-embedded tissues (6). Thus, the immunohistochemical detection
of p53 protein suggests an underlying mutation in the gene
(7). It has been previously
demonstrated that aberrant nuclear expression of p53 protein is
associated with hemizygous p53 deletion in multiple myeloma
(8,9)
and chronic lymphocytic leukemia (10). In MDS, immunohistochemical staining
for TP53 protein in bone marrow trephine biopsies has revealed a
strong correlation with TP53 mutation status (11–13).
A large number of previous studies have reported on
the strong prognostic significance of the immunohistochemical
detection of p53 protein in tumor pathology; however, few studies
have evaluated its prognostic value in MDS (14–16), and
certain studies primarily focused on low-risk MDS with del(5q)
(11,17).
In the present study, p53 immunoreactivity (p53-IR)
was investigated in bone marrow biopsies (BMBs) of patients with
MDS that underwent bone marrow transplantation (BMT), with the aim
of determining its association with clinical and histopathological
data.
Patients and methods
A total of 18 patients that were admitted to the
Division of Haematology (Città della Salute e della Scienza and
University of Turin, Italy) from December 1994 to June 2005, with a
diagnosis of MDS were retrospectively examined. There were 6
females and 12 males; the mean age was 50.5 years (range, 32–64).
Diagnosis of MDS was performed according to the World Health
Organization (WHO) criteria (1). In
total, 5 patients had refractory cytopenia with multilineage
dysplasia (RCMD) and 13 patients had refractory anemia with excess
blasts, type 2 (RAEB-2). Hemoglobin level, white blood cell (WBC)
and platelet counts were assessed from peripheral blood at
diagnosis. All patients underwent allogeneic bone marrow
transplantation. General informed consent was obtained according to
the guidelines of the Ethics Committee, Città della Salute e della
Scienza and University of Turin. Samples were numerically
identified, maintaining patients' anonymity.
BM morphology and
immunohistochemistry
Serial sections (3-µm thick) from Bouin's
solution-fixed, paraffin-embedded BMBs were stained with
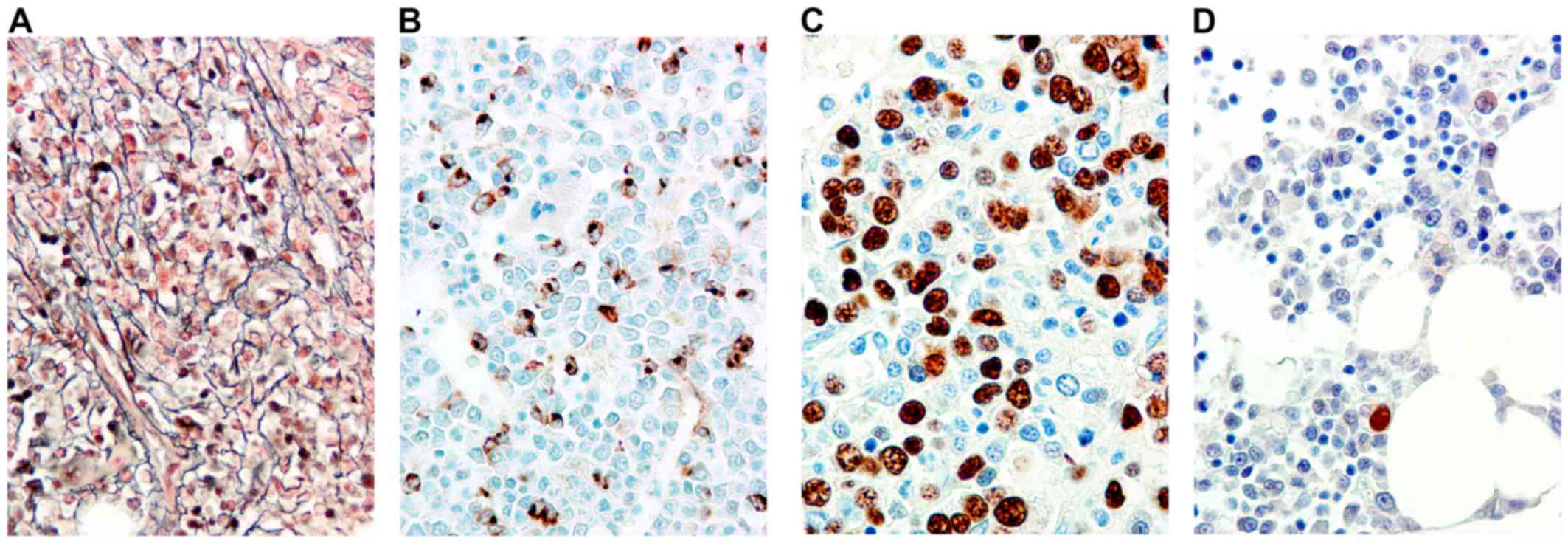
hematoxylin-eosin, Dominici, Perls and reticulin (Fig. 1A) and immunostained with an automated
stainer device (Leica BOND III, Leica Biosystems, Melbourne Pty
Ltd, Mount Waverley VIC 3149, Australia) using a polyclonal
antibody against myeloperoxidase (cat. no. A0398; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA; dilution, 1:1,000), and
monoclonal antibodies against glycophorin A (clone JC159, #M0819;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA; dilution,
1:50), CD61 (clone 2f2; cat. no. 760-4249; Ventana Medical Systems,
Tucson, AZ, USA; undiluted), CD34 (Fig.
1B) (clone QBEnd/10; cat. no. NCL-L-END; Novocastra; Leica
Microsystems, Milton Keynes, UK; dilution, 1;50) and p53 (clone
DO7, #NCL-L-p53-DO7; Novocastra; Leica Microsystems, Milton Keynes,
UK; dilution, 1:100) at room temperature for 15 min.
Paraffin sections (3-µm thick) on SuperFrost
microscope slides were processed with an automated stainer device
(Leica BOND III, Leica Microsystems) and pretreated for 30 min in
citrate buffer (pH 6.0; Bond Epitope Retrieval Solution 1, Leica
Microsystems). The DO-7 antibody was applied at a 1:100 dilution
for 30 min at room temperature and detected using a Bond Polymer
Refine Detection kit (cat. no. DS9800; Leica Microsystems)
according to the manufacturer's protocol. The percentage of cells
with intense (p53++) nuclear staining was calculated examining
≥1,000 hematopoietic cells at a high magnification (x40) using a
standard light microscope. The cut off for positivity (13) was 5% of stained cells (Fig. 1C and D).
Statistical analysis
The association between p53 immunoreactivity
(p53-IR) and clinical or hematological parameters was assessed
using one-way analysis of variance and the Fisher's exact test.
Univariate survival analyses were based on Kaplan-Meier
product-limit estimates of survival distribution, and differences
between survival curves were tested using the log-rank test.
Overall survival (OS) was calculated from the date of diagnosis to
the date of the last observation or death. All analyses were
performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between p53-IR, clinical
and hematological features and bone marrow histology
Positive p53-IR was detected in 7/18 (38.9%)
patients. Significant associations were detected between p53-IR and
patient age (mean age, 57.3 years for positive p53-IR patients vs.
46.4 years for negative p53-IR patients; P=0.005) and the pattern
of BM fibrosis, in which, of the 8 patients with diffuse fibrosis,
6 (75%) were p53-IR-positive compared with 1 (16.7%) of the 6
patients with focal fibrosis (P=0.03). A trend toward significance
was found for sex, (positive p53-IR in 67% of females vs. 25% of
males; P=0.08), BM cellularity (mean BM cellularity, 81.4% for
p53-IR-positive patients vs. 64.5% for p53-IR-negative patients;
P=0.1) and the degree of BM fibrosis (44.4% of WHO-MF1 and 60% of
WHO-MF2 cases were p53-IR-positive, whereas no WHO-MF0 cases were
positive; P=0.1). No association was found for hemoglobin level,
white blood cell (WBC) and platelet counts, percentage of CD34
positive blasts and MDS type; however, the rate of positive p53-IR
was higher in patients with RAEB2 (46.2%) than in those with RCMD
(20%). The results are summarized in Table I.
 | Table I.Association between p53-IR and
clinical and histopathological bone marrow features. |
Table I.
Association between p53-IR and
clinical and histopathological bone marrow features.
|
| P53-IR positive
(N=7) | P53-IR negative
(N=11) |
|
|---|
|
|
|
|
|
|---|
| Variable | N | Mean ± SD | Mean ± SD | P-valuea |
|---|
| Age, years | 18 | 57.3±4.6 | 46.4±8 | <0.01 |
| Hb level, g/dl | 18 | 8.38±1.8 | 9.06±1.70 | 0.40 |
| WBC count,
×109/l | 18 | 3.421±2.989 | 4.380±3.130 | 0.60 |
| Plt count,
×109/l | 18 | 88.7±99.1 | 119.5±81.1 | 0.50 |
| BM cellularity,
% | 18 | 81.4±10.7 | 64.5±27.3 | 0.10 |
| BM blasts, % | 18 | 13.1±5.9 | 11.4±7.7 | 0.60 |
|
| Variable | Ν | N (%) | N (%) | P-valueb |
|
| Sex |
|
|
|
|
| Male | 12 | 3 (25) | 9 (75) |
|
|
Female | 6 | 4 (66.7) | 2 (33.3) | 0.08 |
| MDS type |
|
|
|
|
| RCMD | 5 | 1 (20) | 4 (80) |
|
|
RAEB-2 | 13 | 6 (46.2) | 7 (53.8) | 0.30 |
| BM fibrosis WHO
grade |
|
|
|
|
| MF-0 | 4 | 0 (0) | 4 (100) |
|
| MF-1 | 9 | 4 (44.4) | 5 (55.6) | 0.10 |
| MF-2 | 5 | 3 (60) | 2 (40) |
|
| BM fibrosis
pattern |
|
|
|
|
|
Focal | 6 | 1 (16.7) | 5 (83.3) |
|
|
Diffuse | 8 | 6 (75) | 2 (25) | 0.03 |
Associations between p53-IR,
clinicopathological parameters and overall survival
At the time of analysis, 13 mortalities (72% of
patients) had occurred, and 5 patients (28%) were alive (censored).
The mean OS for the whole series was 73 months (median, 18; range,
2–216). OS was significantly associated with patient age: At 5-year
follow-up, 55% of patients younger <51 years were alive compared
to 44% of older patients (P=0.01). OS was also associated with
hemoglobin level (5 year OS was 73% for patients with hemoglobin
>8 g/dl vs. 14% for patients with hemoglobin ≤8 g/dl; P=0.04),
the type of MDS (5 year OS was 80% for patients with RCMD vs. 38%
for patients with RAEB-2; P=0.05), the degree of BM fibrosis
(Fig. 1A) (all patients without
fibrosis were alive after 5 years vs. 34% of patients with WHO
MF1/2; P=0.006), the number of BM CD34-positive blasts (Fig. 1B) (80% of patients with <5% BM
blasts were alive after 5 years vs. 38% of those with >5% BM
blasts; P=0.05). The median survival for patients with a negative
p53-IR (Fig. 1D) was 105 months vs.
8 months for patients with a positive p53-IR (Fig. 1C) (P=0.1). No difference in OS was
found for sex, WBC and platelet counts. The results are summarized
in Table II.
 | Table II.Correlation between clinical and
histopathological bone marrow features and p53-IR with overall
survival. |
Table II.
Correlation between clinical and
histopathological bone marrow features and p53-IR with overall
survival.
| Variable | N | Median overall
survival, months | 1-year overall
survival rate, % | 5-year overall
survival rate, % | P-valuea |
|---|
| Whole series | 18 | 18 | 61 | 49 |
|
| Sex |
|
|
|
|
|
| Male | 12 | 75 | 67 | 58 |
|
|
Female | 6 | 8.2 | 50 | 33 | 0.60 |
| Age, years |
|
|
|
|
|
| ≤51 | 9 | 108 | 66 | 55 |
|
|
>51 | 9 | 13 | 55 | 44 | 0.01 |
| WBCs,
×109/l |
|
|
|
|
|
| ≤1.8 | 5 | 12 | 60 | 40 |
|
|
>1.8 | 11 | 9 | 55 | 44 | 0.70 |
| Hb level, g/dl |
|
|
|
|
|
| ≤8 | 7 | 8 | 42 | 14 |
|
|
>8 | 11 | 105 | 73 | 73 |
0.04 |
| Plt count,
×109/l |
|
|
|
|
|
|
≤100 | 10 | 75 | 70 | 60 |
|
|
>100 | 8 | 9 | 50 | 37 |
0.80 |
| MDS type |
|
|
|
|
|
|
RCMD | 5 | 105 | 100 | 80 |
|
|
RAEB-2 | 13 | 12 | 46 | 38 |
0.05 |
| BM fibrosis WHO
grade |
|
|
|
|
|
|
MF-0 | 4 | n.a. | 100 | 100 |
|
|
MF-1/2 | 14 | 12 | 57 | 34 | <0.01 |
| BM fibrosis
pattern |
|
|
|
|
|
|
Focal | 6 | 18 | 67 | 50 |
|
|
Diffuse | 8 | 8.2 | 37.5 | 25 |
0.20 |
| BM CD34 positive
blasts |
|
|
|
|
|
| ≤5 | 5 | 105 | 100 | 80 |
|
|
>5 | 13 | 12 | 46 | 38 |
0.05 |
| P53-IR |
|
|
|
|
|
|
Negative | 11 | 105 | 73 | 55 |
|
|
Positive | 7 | 8 | 57 | 43 |
0.10 |
Discussion
Intense p53 (++) immunoreactivity was detected in
7/18 cases (38.8%), in line with the rate of positivity (44%)
previously reported in RCMD/RAEB2 cases (13) using similar staining and scoring
procedure. Although previous studies used 1 or 2% of intensely
immunostained nuclei as a cut off for p53 positivity (11,17), the
present study used 5% strongly (++) immunopositive nuclei as a cut
off, as it has been demonstrated that in 55 del(5q) patients, all
those with >5% p53++ stained cells in BM biopsies carried the
TP53 mutation (11).
p53 immunopositivity was associated with BM fibrosis
in the current study, in which no patients without BM fibrosis were
p53-IR positive, whereas 50% of those with WHO MF1-2 were, and 75%
of patients with diffuse reticulin fibrosis were p53-IR positive,
by contrast to only 16% of patients with focal fibrosis (P=0.03).
Although no significant association was detected, p53 positivity
was higher in RAEB2 than RCMD, in accordance with large studies
revealing that TP53 mutations were observed mainly in patients with
intermediate-2 or high risk MDS (5).
Therefore, with the limitation due to the number of cases, the
current results indicate that a positive p53-IR at diagnosis is
associated with adverse histological prognostic factors, such as BM
fibrosis, and with clinically more aggressive MDS subtypes.
In addition, OS was associated with fibrosis in the
present study; all patients without evidence of fibrosis in BM
biopsy were alive at the 5-year follow-up, whereas only 34% of
those with WHO MF-1/2 disease were (P=0.006). This finding is in
agreement with large studies demonstrating the clinical relevance
of bone marrow fibrosis in primary myelodysplastic syndromes
(18,19). OS was also associated with the type
of MDS: 80% of patients with RCMD were alive at the 5-year
follow-up, whereas only 38% of those with RAEB2 were (P=0.05).
Notably, in the present series, a positive p53-IR
tended to be associated with a shorter OS: The median survival for
patients with negative p53-IR was 105 months but only 8 months for
those with positive p53-IR (P=0.1). This result is concordant with
studies revealing the strong prognostic value of p53 mutations in
MDS (3,5,12,20).
Mutations in TP53, enhancer of zeste homolog 2, ETS variant 6,
runt-related transcription factor 1 and additional sex combs like 1
transcriptional regulator proteins were found to be predictors of
poor overall survival in patients with MDS; however, only mutations
in the TP53 gene have been clearly associated with poor prognostic
markers, and have been reported to independently predict survival,
primarily in patients with intermediate-2 or high risk MDS
(5). A large single institution
study on 318 patients with MDS revealed that TP53 mutations were
the strongest predictor of outcome in a multivariate model
(12).
The present results emphasize the prognostic value
of the immunohistochemical detection of p53 protein in MDS
(11,14–17). The
finding is particularly relevant, when considering the small number
of cases and the type of therapy of the present series. All
patients underwent allogeneic BM transplantation, by contrast to
the previously reported series, which were primarily concerning
patients with low- or intermediate risk del(5q) MDS treated with
lenalidomide.
Immunohistochemistry is a practical and convenient
method to detect p53 protein overexpression in tumor cells
(6). An association has been
reported between intense p53 nuclear staining and TP53 mutation in
MDS (11,13,17);
furthermore, patients without TP53 mutations did not exhibit
intense p53 protein staining, conferring a good negative predictive
value for IHC (12). Therefore, the
acceptable sensitivity of p53 immunostaining in predicting for TP53
mutations is encouraging, and the methodology is routine in
diagnostic laboratories.
In conclusion, our findings suggest that the
evaluation of p53-IR on BMBs of patients with MDS may be introduced
in the histopathological work-up of the disease.
Acknowledgements
The present study was supported by grants from the
Ministero Italiano dell'Università e Ricerca Scientifica (MIUR ex
60%). This study was presented in part at the 19th World Congress
on Advances in Oncology and 17th International Symposium on
Molecular Medicine, 9–11 October 2014, Athens, Greece.
References
|
1
|
Brunning RD, Orazi A, Germing U, Le Beau
MM, Porwit A, Bauman I, Vardiman JW and Hellstrom-Lindberg E:
Myelodysplastic syndromes/neoplasms, overviewWHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo
E, Harris NL, Jaffe ES, Pileri S, Stein H, Thiele J and Vardiman
JW: 4th. IARC Press; Lyon: pp. 88–93. 2008
|
|
2
|
Nagoshi H, Horiike S, Kuroda J and
Taniwaki M: Cytogenetic and molecular abnormalities in
myelodysplastic syndrome. Curr Mol Med. 11:678–685. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaneko H, Misawa S, Horiike S, Nakai H and
Kashima K: TP53 mutations emerge at early phase of myelodysplastic
syndrome and are associated with complex chromosomal abnormalities.
Blood. 85:2189–2193. 1995.PubMed/NCBI
|
|
4
|
Christiansen DH, Andersen MK and
Pedersen-Bjergaard J: Mutations with loss of heterozygosity of p53
are common in therapy-related myelodysplasia and acute myeloid
leukemia after exposure to alkylating agents and significantly
associated with deletion or loss of 5q, a complex karyotype and a
poor prognosis. J Clin Oncol. 19:1405–1413. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bejar R, Stevenson K, Abdel-Wahab O,
Galili N, Nilsson B, Garcia-Manero G, Kantarjian H, Raza A, Levine
RL, Neuberg D and Ebert BL: Clinical effect of point mutations in
myelodysplastic syndromes. N Engl J Med. 364:2496–2506. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerns BM, Jordan PA, Moore MB, Humphrey
PA, Berchuck A, Kohler MF, Iglebart RC Jr, Bast JD and Marks JR:
p53 overexpression in formalin-fixed, paraffin-embedded tissue
detected by immunohistochemistry. J Histochem Cytochem.
40:1047–1051. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iggo R, Gatter K, Bartek J, Lane D and
Harris AL: Increased expression of mutant forms of p53 oncogene in
primary lung cancer. Lancet. 335:675–679. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang H, Yeung J, Qi C and Xu W: Aberrant
nuclear p53 protein expression detected by immunohistochemistry is
associated with hemizygous P53 deletion and poor survival for
multiple myeloma. Br J Haematol. 138:324–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MH, Qi CX, Saha MN and Chang H: p53
nuclear expression correlates with hemizygous TP53 deletion and
predicts an adverse outcome for patients with relapsed/refractory
multiple myeloma treated with lenalidomide. Am J Clin Pathol.
137:208–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang H, Jiang AM and Qi CX: Aberrant
nuclear p53 expression predicts hemizygous 17p (TP53) deletion in
chronic lymphocytic leukemia. Am J Clin Pathol. 133:70–74. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jädersten M, Saft L, Smith A,
Kulasekararaj A, Pomplun S, Göhring G, Hedlund A, Hast R,
Schlegelberger B, Porwit A, et al: TP53 mutations in low-risk
myelodysplastic syndromes with del(5q) predict disease progression.
J Clin Oncol. 29:1971–1979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kulasekararaj AG, Smith AE, Mian SA,
Mohamedali AM, Krishnamurthy P, Lea NC, Gäken J, Pennaneach C,
Ireland R, Czepulkowski B, et al: TP53 mutations in myelodysplastic
syndrome are strongly correlated with aberrations of chromosome 5,
and correlate with adverse prognosis. Br J Haematol. 160:660–672.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller-Thomas C, Rudelius M, Rondak IC,
Haferlach T, Schanz J, Huberle C, Schmidt B, Blaser R, Kremer M,
Peschel C, et al: Response to azacitidine is independent of p53
expression in higher-risk myelodysplastic syndromes and secondary
acute myeloid leukemia. Haematologica. 99:e179–e181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orazi A, Cattoretti G, Heerema NA, Sozzi
G, John K and Neiman RS: Frequent p53 overexpression in therapy
related myelodysplastic syndromes and acute myeloid leukemias: An
immunohistochemical study of bone marrow biopsies. Mod Pathol.
6:521–525. 1993.PubMed/NCBI
|
|
15
|
Kitagawa M, Yoshida S, Kuwata T, Tanizawa
T and Kamiyama R: p53 expression in myeloid cells of
myelodysplastic syndromes. Association with evolution of overt
leukemia. Am J Pathol. 145:338–444. 1994.PubMed/NCBI
|
|
16
|
Brynes RK, Wilson CS, Kim AB and McCourty
A: Expression of p53, MDM2, p21waf1, bcl-2, and retinoblastoma gene
proteins in myelodysplastic syndrome after autologous bone marrow
transplantation for lymphoma. Mod Pathol. 10:1120–1127.
1997.PubMed/NCBI
|
|
17
|
Saft L, Karimi M, Ghaderi M, Matolcsy A,
Mufti GJ, Kulasekararaj A, Göhring G, Giagounidis A, Selleslag D,
Muus P, et al: p53 protein expression independently predicts
outcome in patients with lower-risk myelodysplastic syndromes with
del(5q). Haematologica. 99:1041–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Porta MG Della, Malcovati L, Boveri E,
Travaglino E, Pietra D, Pascutto C, Passamonti F, Invernizzi R,
Castello A, Magrini U, et al: Clinical relevance of bone marrow
fibrosis and CD34-positive cell clusters in primary myelodysplastic
syndromes. J Clin Oncol. 27:754–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cazzola M and Malcovati L: Prognostic
classification and risk assessment in myelodysplastic syndromes.
Hematol Oncol Clin North Am. 24:459–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugimoto K, Hirano N, Toyoshima H, Chiba
S, Mano H, Takaku F, Yazaki Y and Hirai H: Mutations of the p53
gene in myelodysplastic syndrome (MDS) and MDS-derived leukemia.
Blood. 81:3022–3026. 1993.PubMed/NCBI
|