Introduction
Prognostic information is essential when choosing
between treatment regimens with different levels of intensity, and
when stratifying patients who participate in clinical studies.
Often, different items, which contribute information, are combined
into a clinically useful tool, e.g., a staging system or prognostic
score (1). In patients with
progressive cancer, the prognosis changes during the disease
trajectory (2). If an assessment
could be based on factors that are easy to measure and inexpensive,
clinical decision-making would be improved, particularly in
low-income countries with limited health care resources. In this
context, the Glasgow prognostic score (GPS) might be an excellent
tool (3). It is based on measurement
of the levels of serum albumin and C-reactive protein (CRP),
reflects inflammatory processes that may perpetuate tumor
progression, and has been shown to correlate with survival in
patients with lung, gastrointestinal, prostate and renal cancer
(4–6). Surprisingly little research has been
performed regarding its potential role in patients who receive
palliative radiotherapy. As the role of palliative radiotherapy in
patients with very short survival has recently come under scrutiny
(7–9), in the present study, the ability of GPS
to predict survival in a large contemporary cohort of patients was
examined.
Patients and methods
A retrospective analysis of the records of 873
consecutive patients with metastatic or otherwise incurable cancer
who received palliative radiotherapy at a single institution
(Nordland Hospital, Bodø, Norway) was performed. Due to their
different biological behavior, hematological and primary brain
malignancies were not included. Treatment was initiated during the
time period between 20 June 2007 (the date of opening of the
institution's radiotherapy facility) and 31 December 2011. Medical
records and treatment details were abstracted from the hospital's
electronic patient record (EPR) system. The survival status and
date of death, or last follow-up, of the patients were obtained
from the EPR during September 2014, resulting in at least 2.5 years
of follow-up for surviving patients. IBM SPSS Statistics 21.0 (IBM
SPSS, Armonk, NY, USA) was used to evaluate the association between
survival, GPS and other prognostic factors. Only blood test results
obtained within 1 week prior to the radiotherapy were considered.
For albumin, the institutional lower limit was 36 g/l. The cut-off
for CRP was 8 mg/l. Overall, 703 patients had available blood
tests, and were eligible for the present study. The Eastern
Cooperative Oncology Group (ECOG) performance status (PS) was
registered routinely at the time of consultation in conjunction
with radiotherapy (10). Actuarial
survival curves from the first day of radiotherapy were generated
using the Kaplan-Meier method, and compared using the log-rank
test. For multivariate analysis of survival, Cox regression
analysis was used (the forward stepwise method). All factors with a
significant P-value in univariate log-rank tests were carried
forward to multivariate regression analysis. Associations between
different variables of interest were assessed with the Chi-square
and Fisher exact probability tests. In two-sided tests, P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics in 703
patients
The median age was 68 years (range, 31–95 years).
The median time from first cancer diagnosis to palliative
radiotherapy was 2 years (range, 0–30 years). In patients with
distant metastases, the median interval between diagnosis of
metastatic disease and palliative radiotherapy was 7 months (range,
0–149 months). Common treatment indications included skeletal
metastases (57%), brain metastases (16%), thoracic symptoms from
lung cancer (12%), and lymph node metastases (9%). The most common
fractionation regimen was 3 Gy × 10 (45%), followed by 5–7
fractions of 4 Gy (22%) and single-fraction 8 Gy × 1 (8.5 Gy ×2 for
lung cancer) in 15% of the cases. Stereotactic radiotherapy was not
available. The majority of the patients had prostate cancer (26%),
non-small cell lung cancer (NSCLC, 22%) or breast cancer (13%) as
primary tumors.
GPS reflects prognostically important
disease characteristics
A total of 34% of the patients had normal CRP and
albumin scores (GPS 0). Of the total patients, 19% had abnormal CRP
and albumin (GPS 2). The remaining 47% of the patients had GPS 1
(in 96% of cases resulting from an elevated level of CRP; in 4%,
due to a low level of albumin). As shown in Table I, the majority of the patients with
GPS 0 had a good PS (ECOG 0 or 1), whereas most patients with GPS 2
had a poor PS (ECOG 3 or 4; P=0.0001). Significant differences were
also observed regarding the primary tumor type. Primary tumors that
were frequently associated with GPS 2 were pancreatic cancer (40%
of these patients had GPS 2), bladder cancer (30%), colorectal
cancer (28%), and NSCLC (26%). The pattern of metastatic disease
also correlated with GPS. Patients with GPS 2 had markedly higher
rates of adrenal gland, lung and liver metastases. Increasing
levels of GPS were correlated with an increasing likelihood of
documented disease progression outside of the radiotherapy target
volumes(s) at the time of the last assessment prior to radiotherapy
(P=0.0001). Anemia was more common in patients with a higher GPS
(P=0.0001). Comparable correlations were detected for leukocytosis
and high platelet counts (P=0.0001). Increasing GPS was also
associated with a low level of serum creatinine, a surrogate marker
of reduced muscle mass, or cachexia (P=0.0001).
 | Table I.Baseline characteristics stratified by
GPS scores of the patients (n=703). |
Table I.
Baseline characteristics stratified by
GPS scores of the patients (n=703).
| Parameter | 0 | % | 1 | % | 2 | % | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
Female | 90 | 37 | 114 | 35 | 48 | 37 |
|
| Male | 151 | 63 | 216 | 65 | 83 | 63 | 0.77 |
| Age, years |
|
|
|
|
|
|
|
|
<60 | 47 | 20 | 71 | 21 | 24 | 18 | 0.7 |
| ≥80 | 38 | 16 | 44 | 13 | 20 | 15 | 0.46 |
| ECOG PS |
|
|
|
|
|
|
|
| 0 | 41 | 17 | 35 | 11 | 2 | 2 |
|
| 1 | 111 | 46 | 98 | 30 | 10 | 8 |
|
| 2 | 63 | 26 | 124 | 37 | 46 | 35 |
|
| 3 | 25 | 10 | 61 | 18 | 63 | 48 |
|
| 4 |
0 | 0 | 13 | 4 | 10 | 8 | 0.0001 |
| Type of cancer |
|
|
|
|
|
|
|
| Prostate
cancer | 91 | 38 | 71 | 21 | 21 | 16 |
|
| Breast
cancer | 43 | 18 | 31 | 9 | 15 | 11 |
|
| Non-small
cell lung cancer | 34 | 14 | 82 | 25 | 41 | 31 |
|
| Small
cell lung cancer | 15 | 6 | 24 | 7 |
4 | 3 |
|
|
Colorectal cancer | 10 | 4 | 23 | 7 | 13 | 10 |
|
|
Pancreatic cancer |
1 | 0 |
5 | 2 |
4 | 3 |
|
| Bladder
cancer |
7 | 3 | 24 | 7 | 13 | 10 |
|
| Malignant
melanoma |
9 | 4 | 11 | 3 | 2 | 2 |
|
| Kidney
cancer | 11 | 5 | 35 | 11 | 8 | 6 |
|
| Other
primary tumor | 20 | 8 | 25 | 8 | 10 | 8 | 0.0001 |
| Metastases |
|
|
|
|
|
|
|
|
Bonea | 162 | 68 | 222 | 67 | 102 | 78 | 0.06 |
|
Braina | 50 | 21 | 67 | 20 | 22 | 17 | 0.61 |
|
Livera | 40 | 17 | 77 | 23 | 37 | 28 | 0.028 |
|
Lunga | 41 | 17 | 97 | 29 | 46 | 35 | 0.0001 |
|
Adrenala | 14 | 6 | 38 | 11 | 25 | 19 | 0.0001 |
| No
distant | 28 | 4 | 34 | 5 |
8 | 1 | 0.23 |
| Progressive disease
outside of RT field | 97 | 42 | 189 | 58 | 90 | 73 | 0.0001 |
| Anemiab | 101 | 42 | 223 | 68 | 116 | 89 | 0.0001 |
|
Leukocytosisc | 31 | 13 | 83 | 25 | 56 | 44 | 0.0001 |
|
Thrombocytosisd | 34 | 14 | 105 | 33 | 50 | 40 | 0.0001 |
| Serum
creatinine |
|
|
|
|
|
|
|
| Lowe | 16 | 2 | 49 | 7 | 36 | 5 | 0.0001e |
| Highe | 27 | 4 | 58 | 8 | 19 | 3 |
|
| No previous
systemic therapy | 88 | 39 | 131 | 42 | 58 | 48 | 0.26 |
GPS reflects resource utilization and
predicts early death
As shown in Table
II, GPS was associated with the utilization of palliative care
resources, the number of blood transfusions and intravenous
treatment with antibiotics. A larger proportion of the patients
with GPS 2 were unable to complete their prescribed course of
radiotherapy. One-third of patients with GPS 2 received palliative
radiotherapy in the terminal phase (final month of life). Patients
with GPS 2 were less often treated with long-course radiotherapy,
defined as ≥10 fractions (46%, compared with 62% in the subgroup
with GPS 1, and 72% of those with GPS 0; P=0.0001).
 | Table II.Resource utilization and treatment
completion stratified by GPS scores of the patients (n=703). |
Table II.
Resource utilization and treatment
completion stratified by GPS scores of the patients (n=703).
| Endpoint | 0 | % | 1 | % | 2 | % | P-value |
|---|
| Received blood
transfusion during RT | 2 | 1 | 17 | 5 | 15 | 12 | 0.0001 |
| Received iv
antibiotics during RT | 7 | 3 | 34 | 11 | 28 | 23 | 0.0001 |
| Care by palliative
team during RT | 38 | 17 | 75 | 24 | 42 | 37 | 0.0001 |
| Pain management
with continuous infusion pump | 2 | 1 | 14 | 4 | 17 | 13 | 0.0001 |
| Incomplete RT | 4 | 2 | 21 | 6 | 17 | 13 | 0.0001 |
| RT during last
month of life | 8 | 3 | 33 | 10 | 43 | 33 | 0.0001 |
Survival
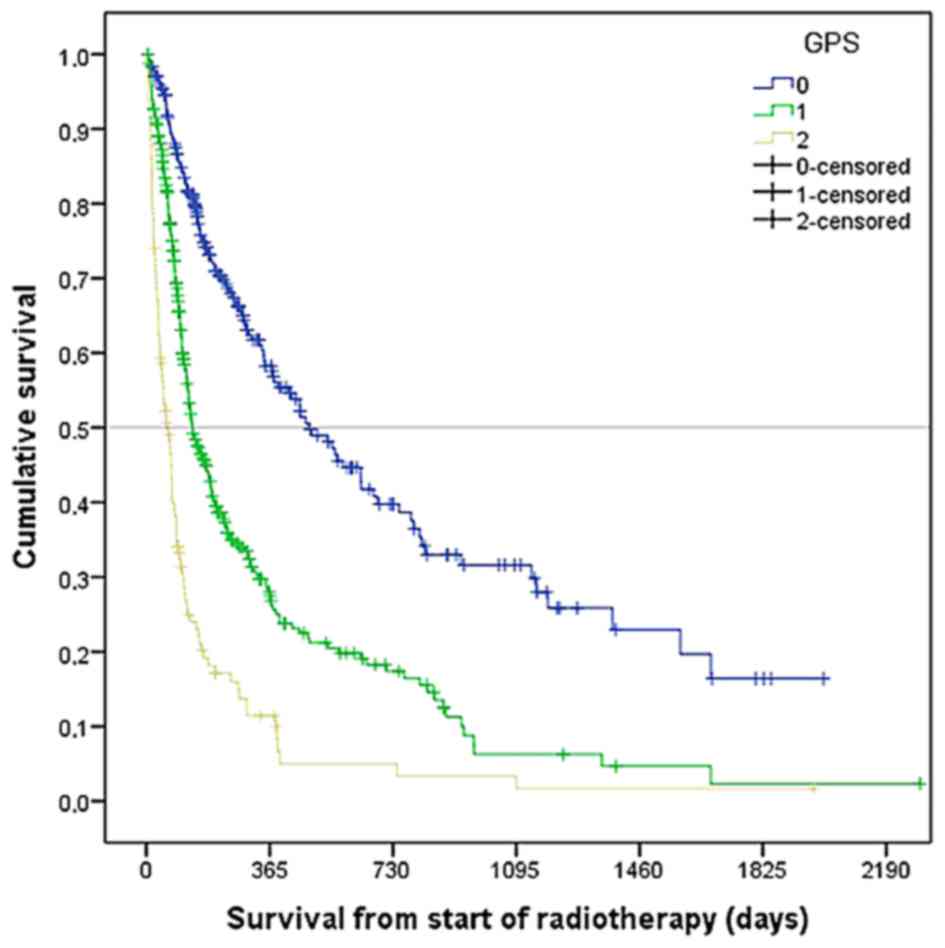
The median survival rate was 183 days (6 months). As
shown in Fig. 1, GPS significantly
correlated with survival (median 479, 136, and 61 days,
respectively, for GPS 0, 1 and 2; P=0.0001). Therefore GPS was
entered into a multivariate Cox regression model, together with all
the other prognostic factors, which achieved a P-value<0.05 in
univariate log-rank tests [age as a continuous variable, ECOG PS,
primary tumor type, anemia, leukocytosis, low serum creatinine,
progressive disease outside of target volume(s), brain metastases,
liver metastases, lung metastases, bone metastases, and adrenal
gland metastases]. Seven out of these 13 variables were revealed to
be independent prognostic factors for survival: GPS, ECOG PS, brain
metastases, liver metastases, bone metastases, progressive disease
outside of target volume(s), and leukocytosis (all with
P≤0.001).
On the one hand, the median survival of patients
with GPS 2 was short, and administration of radiotherapy during the
last month of life was not unusual. On the other hand, survival in
this subgroup was too heterogeneous to conclude that all these
patients were poor candidates for radiotherapy. An appreciable
number of them survived long enough to benefit from and experience
symptom improvement. Thus, additional variables are required in
order to define a subgroup with uniformly short survival. As
mentioned above, these variables should be easy to assess and
inexpensive in order to decide upon which model would be applicable
to practice settings in countries with limited resources. Factoring
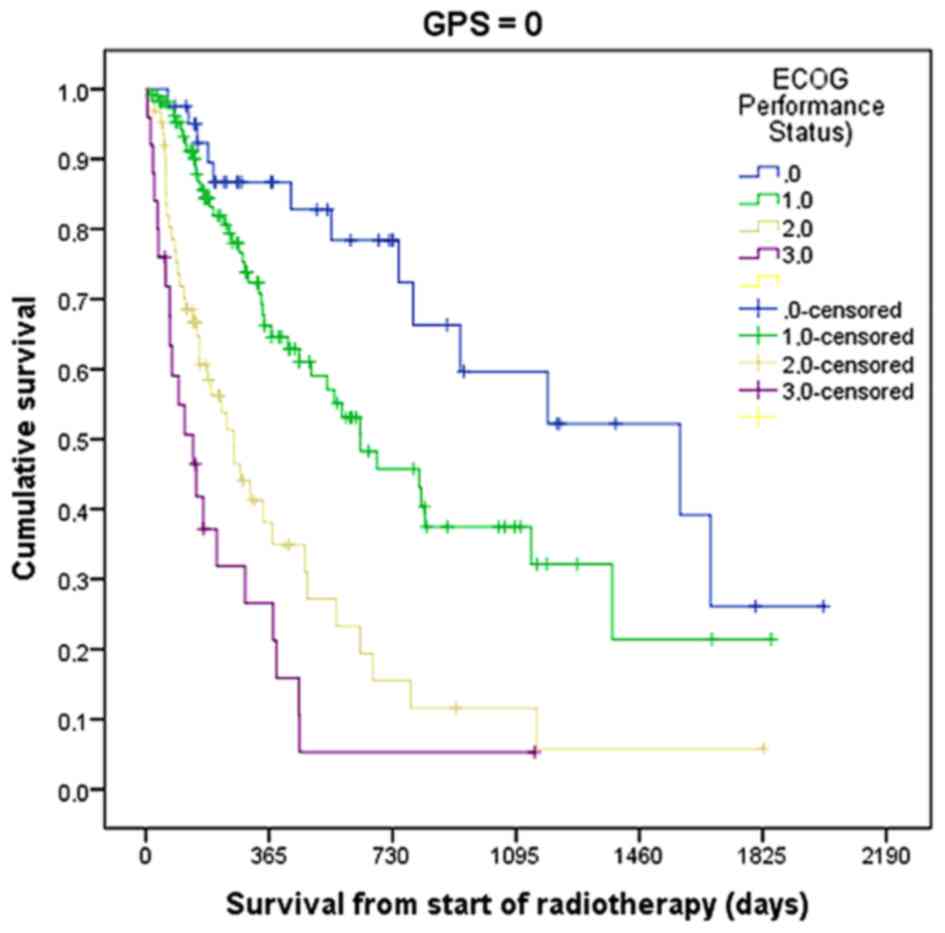
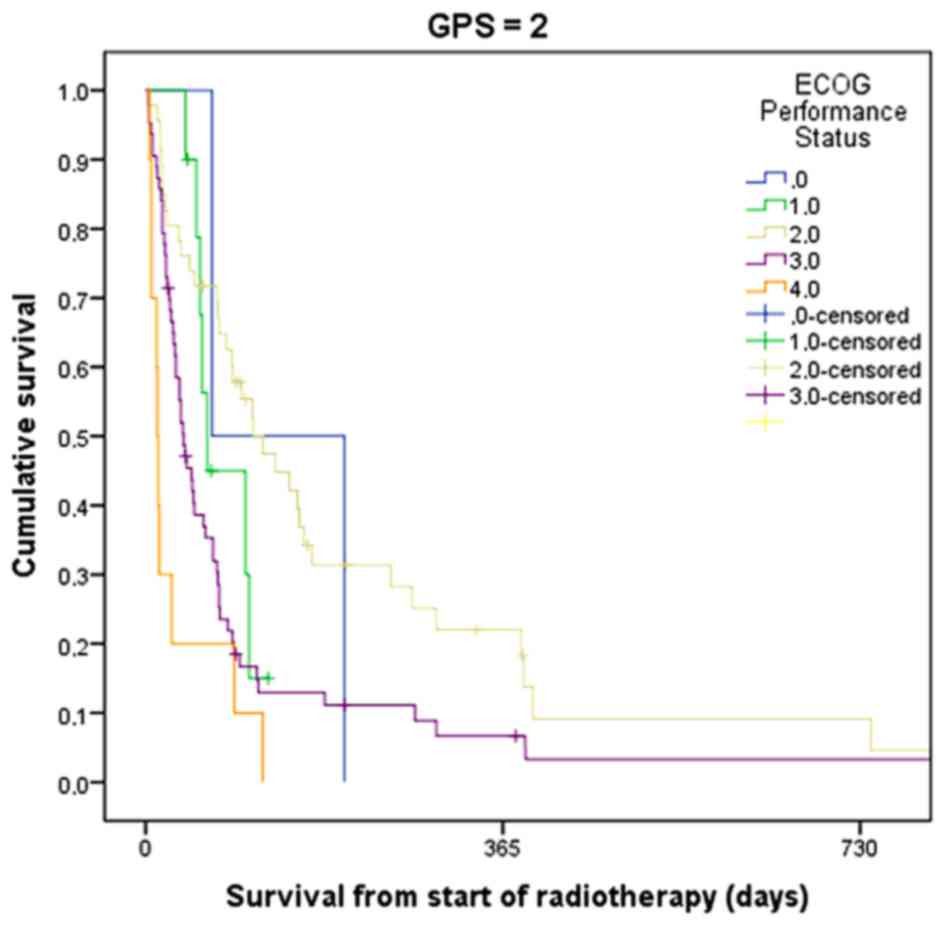
in ECOG PS was clearly a way forward. Figs. 2 and 3
show the usefulness of combining GPS and ECOG PS, at least in
patients with GPS 0 and 1. However, in the relevant target group of
patients with GPS 2, the survival data did not reveal significant
differences (Fig. 4). Table III shows the median survival of
patients with different ECOG PS stratified by GPS. The two groups
with the shortest survival rates were patients with ECOG PS 3 and
GPS 2, and patients with ECOG PS 4, irrespective of GPS. The second
variable not requiring any additional resources (such as, e.g.,
imaging) was leukocytosis. This may be evaluated from the drawing
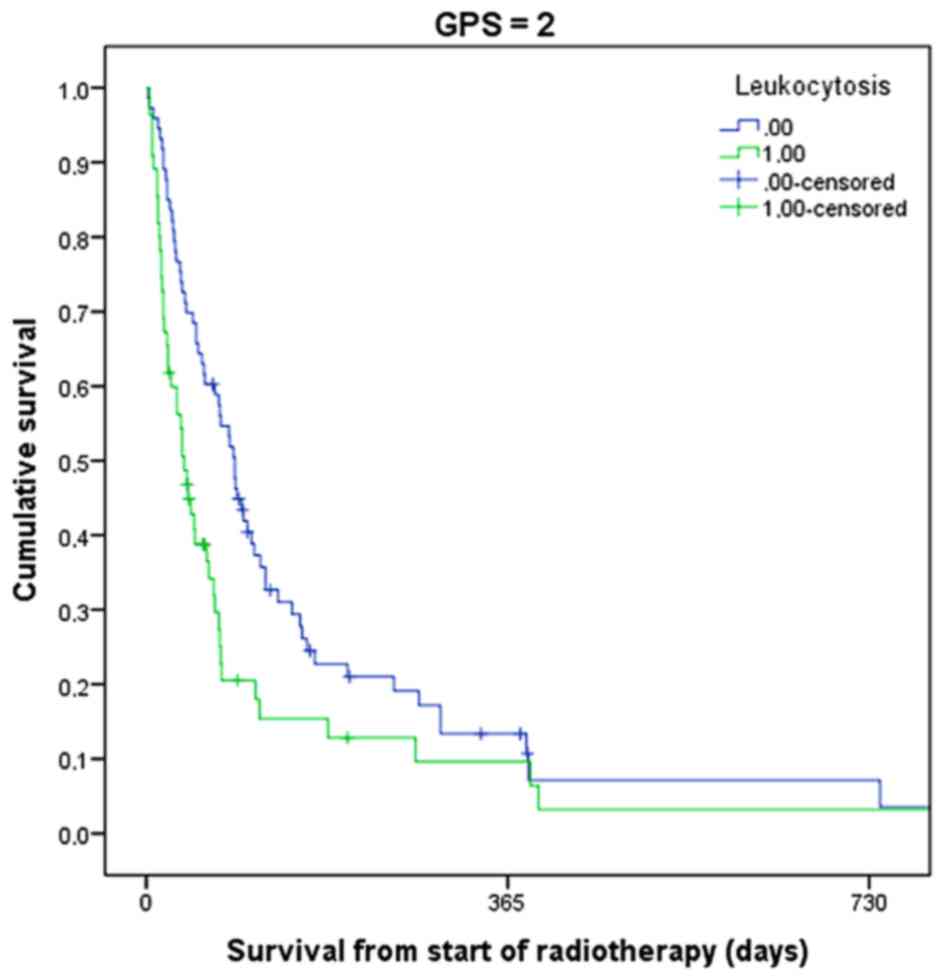
of the same blood required to assign GPS. As shown in Fig. 5, patients with GPS 2 and leukocytosis
had a median survival of 38 days, as compared with 89 days in those
with a normal leukocyte count (P=0.007). Only 20% of the patients
with three abnormal biomarkers were alive after 3 months.
Comparable associations were observed in patients with GPS 1
(median, 94 vs. 192 days) and GPS 0 (median, 162 vs. 635 days).
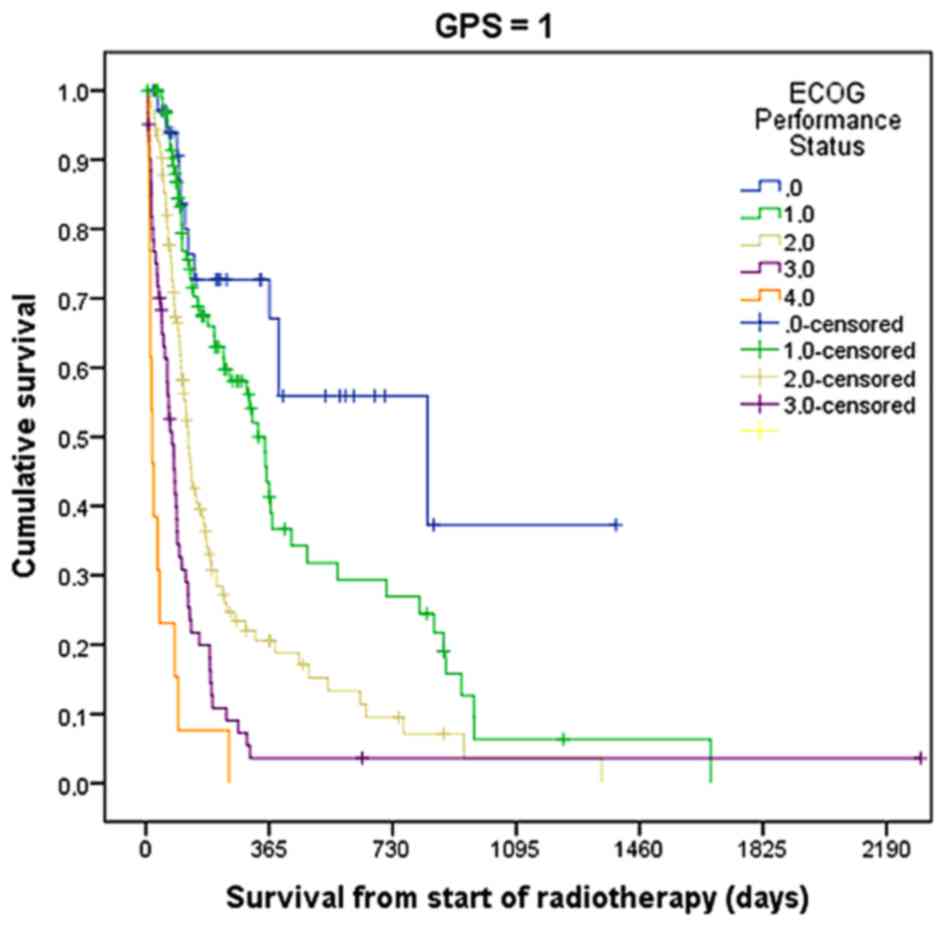 | Figure 3.Actuarial overall survival following
palliative radiotherapy in patients with GPS 1 stratified by ECOG
performance status (P=0.001, log-rank test). The number of patients
was 35, 98, 124, 61, and 13 for ECOS performance statuses of 0, 1,
2, 3, and 4, respectively. GPS, Glasgow prognostic score; ECOG,
Eastern Cooperative Oncology Group. |
 | Figure 4.Actuarial overall survival following
palliative radiotherapy in patients with GPS 2 stratified by ECOG
performance status (P=0.3, log-rank test). The number of patients
was 2, 10, 46, 63, and 10, for ECOS performance statuses of 0, 1,
2, 3, and 4, respectively. GPS, Glasgow prognostic score; ECOG,
Eastern Cooperative Oncology Group. |
 | Table III.Median survival (days, 95% confidence
interval) in patients with different PSs stratified by GPS scores
(n=702). |
Table III.
Median survival (days, 95% confidence
interval) in patients with different PSs stratified by GPS scores
(n=702).
| ECOS PS | No. of
patients | 0 | 1 | 2 |
|---|
| 0 | 78 | 1,580
(686–2,474) | 833 (58–1,608) | 68 (n/a; only 2
patients) |
| 1 | 219 | 636 (372–900) | 332 (275–389) | 63 (49–77) |
| 2 | 233 | 261 (163–359) | 126 (107–145) | 120 (70–170) |
| 3 | 149 | 140 (57–223) | 78 (57–99) | 39 (26–52) |
| 4 | 23 | No patients | 19 (7–31) | 12 (9–15) |
Models with a different CRP
cut-off
GPS does not take into account the magnitude of CRP
increase: Assignment is based solely on normal vs. increased CRP.
Therefore, the analyses in the present study were expanded, and
different levels of an increase in CRP (30, 60 or 90 mg/l) were
examined. However, survival prediction did not improve in these
analyses requiring certain levels of an increase in CRP (results
not shown). Albumin levels were less variable. Almost all patients
with low levels of albumin had measurements of 30–35 g/l.
Therefore, stratified analyses could not be meaningfully
performed.
Discussion
Irrespective of the health care setting, increasing
expenses and limited budgets create serious challenges for the
oncology community. Initiatives such as the ‘Choosing Wisely’
campaign (11) are trying to provide
support to stakeholders, who are required to discriminate between
cost-effective interventions and imprudent resource utilization.
Although palliative radiotherapy, in general, is considered
excellent value for money, the large number of available
fractionation regimens and techniques, and the broad spectrum of
survival outcomes, make it difficult to always ensure that the
correct treatment is assigned to the right patient (12). Staging systems and prognostic scores
play an invaluable role when assessing individual patients and
making treatment recommendations, as demonstrated by the broad
adoption of brain metastases scores (13,14).
Particularly in patients with a limited survival expectation,
decision making should not require comprehensive and expensive
restaging, which could also delay the initiation of meaningful
palliative interventions. These considerations are even more
relevant for low-income countries. Widely available routine blood
tests may provide excellent prognostic information, and value for
money (15), since results are
available within a few hours.
During the last decade, an inflammation-based
prognostic score, GPS, has been studied in different oncology
settings, e.g., in chemotherapy for NSCLC and prostate cancer
(3,6). This research suggests that malnourished
cancer patients had a statistically significant higher CRP,
neutrophil-to-lymphocyte ratio and GPS prior to starting the
chemotherapy (16). Eventually, it
was shown that GPS predicts cancer survival independently of the
tumor site (4). Limited data are
available regarding GPS and radiotherapy (17). The latter study focused on the
end-of-life care, and identified that GPS 2 was strongly associated
with the receipt of radiotherapy during the last 30 days of life.
The present study, to the best of the authors' knowledge, is the
first comprehensive analysis of GPS in a large cohort of patients
who received palliative radiotherapy. GPS was assigned
retrospectively, and not used during the time period of actual
patient treatments. Only a minority of patients (34%) had normal
CRP and albumin, i.e., GPS 0. Strong associations between GPS and
ECOG PS were identified, comparable with earlier reports (18). It is tempting to speculate that GPS
is merely a surrogate marker of tumor extent and tumor-host
interactions, since GPS correlated with progression, the pattern of
metastatic disease, anemia, leukocytosis and thrombocytosis.
However, in multivariate analysis of prognostic factors for overall
survival that included all these variables, the added value of GPS
was confirmed. This finding is in line with non-radiotherapy
studies (5,19,20). Due
to its robust prognostic value, low cost and wide availability,
additional studies of GPS in different radiotherapy settings are
recommended.
Previous studies also found significant associations
between GPS and the chemotherapy dose adjustment, the requirement
for granulocyte colony-stimulating factor support, termination of
treatment due to side-effects, and fatal toxicity (21). In the present study, GPS was
associated with more frequent utilization of palliative care
resources, the number of blood transfusions and intravenous
treatment with antibiotics. Compared with patients with GPS 0 or 1,
more patients with GPS 2 were not able to complete their prescribed
course of radiotherapy. A total of 33% of the patients with GPS 2
received treatment during the final month of their life. It is
therefore important to consider short-course (e.g., 8.5 Gy × 2, 4
Gy × 5) and single-fraction (8 Gy) radiotherapy regimens in
patients with GPS 2. The development of prediction models
specifically for the purpose of avoidance of too aggressive
end-of-life care is in its infancy, and, for the time being, is an
unmet requirement (22–24). A previous study suggested that
performance status and GPS, used together, act synergistically,
thus improving the prognostic accuracy (19). Even if this was not entirely
confirmed in the present study, patients with GPS 2 and ECOG PS 3
or 4 had short median survival rates. Possibly, the discrepancy
between the two studies was caused by the small group sizes.
In the present study, it was established that it was
possible to improve the prediction of short survival by combining
leukocytosis and GPS 2. Patients with these two characteristics had
a median survival of >6 weeks. This combination of variables has
not been evaluated previously in the same setting. Considering
cancer-specific survival, Proctor et al (25) demonstrated that the prognostic value
of the GPS improved by the addition of neutrophil and platelet
counts and a high-sensitivity CRP measurement (>3 mg/l). Neither
neutrophil counts nor high-sensitivity CRP assays were part of our
standard work-up during the time period of the present study. In
addition, the platelet count was not significantly associated with
survival.
In conclusion, the present study has revealed that
three widely available, inexpensive blood tests (CRP, albumin, and
leukocyte count) provide clinically relevant prognostic information
for radiation oncologists who decide upon fractionation. Further
studies of the original GPS, and modified variants, are
warranted.
References
|
1
|
Tseng YD, Krishnan MS, Sullivan AJ, Jones
JA, Chow E and Balboni TA: How radiation oncologists evaluate and
incorporate life expectancy estimates into the treatment of
palliative cancer patients: A survey-based study. Int J Radiat
Oncol Biol Phys. 87:471–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Likhacheva A, Pinnix CC, Parikh NR, Allen
PK, McAleer MF, Chiu MS, Sulman EP, Mahajan A, Guha-Thakurta N,
Prabhu SS, et al: Predictors of survival in contemporary practice
after initial radiosurgery for brain metastases. Int J Radiat Oncol
Biol Phys. 85:656–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Comparison of an inflammation-based
prognostic score (GPS) with performance status (ECOG) in patients
receiving platinum-based chemotherapy for inoperable non-small-cell
lung cancer. Br J Cancer. 90:1704–1706. 2004.PubMed/NCBI
|
|
4
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A Glasgow Inflammation Outcome Study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linton A, Pond G, Clarke S, Vardy J,
Galsky M and Sonpavde G: Glasgow prognostic score as a prognostic
factor in metastatic castration-resistant prostate cancer treated
with docetaxel-based chemotherapy. Clin Genitourin Cancer.
11:423–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guadagnolo BA, Liao KP, Elting L, Giordano
S, Buchholz TA and Shih YC: Use of radiation therapy in the last 30
days of life among a large population-based cohort of elderly
patients in the United States. J Clin Oncol. 31:80–87. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy JD, Nelson LM, Chang DT, Mell LK
and Le QT: Patterns of care in palliative radiotherapy: A
population-based study. J Oncol Pract. 9:e220–e227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kapadia NS, Mamet R, Zornosa C, Niland JC,
D'Amico TA and Hayman JA: Radiation therapy at the end of life in
patients with incurable nonsmall cell lung cancer. Cancer.
118:4339–4345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly CM and Shahrokni A: Moving beyond
Karnofsky and ECOG performance status assessments with new
technologies. J Oncol. 2016:61865432016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hahn C, Kavanagh B, Bhatnagar A, Jacobson
G, Lutz S, Patton C, Potters L and Steinberg M: Choosing wisely:
The American Society for Radiation Oncology's top 5 list. Pract
Radiat Oncol. 4:349–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olson RA, Tiwana MS, Barnes M, Kiraly A,
Beecham K, Miller S, Hoegler D and Olivotto I: Use of single-
versus multiple-fraction palliative radiation therapy for bone
metastases: Population-based analysis of 16,898 courses in a
Canadian province. Int J Radiat Oncol Biol Phys. 89:1092–1099.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh
J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, et al:
Diagnosis-specific prognostic factors, indexes, and treatment
outcomes for patients with newly diagnosed brain metastases: A
multi-institutional analysis of 4,259 patients. Int J Radiat Oncol
Biol Phys. 77:655–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
Radiation Therapy Oncology Group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Partl R, Richtig E, Avian A, Berghold A
and Kapp KS: Karnofsky performance status and lactate dehydrogenase
predict the benefit of palliative whole-brain irradiation in
patients with advanced intra- and extracranial metastases from
malignant melanoma. Int J Radiat Oncol Biol Phys. 85:662–666. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan CS, Read JA, Phan VH, Beale PJ, Peat
JK and Clarke SJ: The relationship between nutritional status,
inflammatory markers and survival in patients with advanced cancer:
A prospective cohort study. Support Care Cancer. 23:385–391. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anshushaug M, Gynnild MA, Kaasa S, et al:
Characterization of patients receiving palliative chemo- and
radiotherapy during end of life at a regional cancer center in
Norway. Acta Oncol. 27:1–8. 2014.
|
|
18
|
Leung EY, Scott HR and McMillan DC:
Clinical utility of the pretreatment glasgow prognostic score in
patients with advanced inoperable non-small cell lung cancer. J
Thorac Oncol. 7:655–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laird BJ, Kaasa S, McMillan DC, Fallon MT,
Hjermstad MJ, Fayers P and Klepstad P: Prognostic factors in
patients with advanced cancer: A comparison of clinicopathological
factors and the development of an inflammation-based prognostic
system. Clin Cancer Res. 19:5456–5464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furukawa K, Shiba H, Haruki K, Fujiwara Y,
Iida T, Mitsuyama Y, Ogawa M, Ishida Y, Misawa T and Yanaga K: The
Glasgow prognostic score is valuable for colorectal cancer with
both synchronous and metachronous unresectable liver metastases.
Oncol Lett. 4:324–328. 2012.PubMed/NCBI
|
|
21
|
Gioulbasanis I, Pallis A, Vlachostergios
PJ, Xyrafas A, Giannousi Z, Perdikouri IE, Makridou M, Kakalou D
and Georgoulias V: The Glasgow Prognostic Score (GPS) predicts
toxicity and efficacy in platinum-based treated patients with
metastatic lung cancer. Lung Cancer. 77:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angelo K, Norum J, Dalhaug A, Pawinski A,
Aandahl G, Haukland E, Engljähringer K and Nieder C: Development
and validation of a model predicting short survival (death within
30 days) after palliative radiotherapy. Anticancer Res. 34:877–885.
2014.PubMed/NCBI
|
|
23
|
Gripp S, Mjartan S, Boelke E and Willers
R: Palliative radiotherapy tailored to life expectancy in end-stage
cancer patients: Reality or myth? Cancer. 116:3251–3256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieder C, Angelo K, Dalhaug A, Pawinski A,
Haukland E and Norum J: Palliative radiotherapy during the last
month of life: Predictability for referring physicians and
radiation oncologists. Oncol Lett. 10:3043–3049. 2015.PubMed/NCBI
|
|
25
|
Proctor MJ, Horgan PG, Talwar D, Fletcher
CD, Morrison DS and McMillan DC: Optimization of the systemic
inflammation-based Glasgow prognostic score: A Glasgow Inflammation
Outcome Study. Cancer. 119:2325–2332. 2013. View Article : Google Scholar : PubMed/NCBI
|